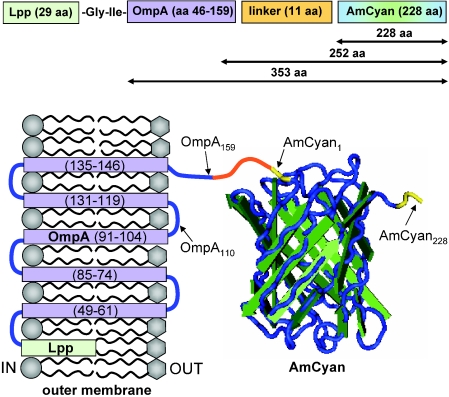
FIG. 3.
Schematic of the expected structure of the Lpp-OmpA-AmCyan fusion in the outer membrane of E. coli. This chimeric fusion is composed of two polypeptide domains in series (a portion of the outer membrane protein A, labeled OmpA, and the entire sequence of the cyan-fluorescent protein AmCyan), which are joined to one another by an 11-amino-acid linker and anchored into the outer membrane by a lipoprotein (Lpp). The total length of the fusion is 384 amino acids. The numbers across the top (353, 252, and 228) correspond to the lengths of the polypeptides described by the wormlike chain and freely joined chain models in Fig. 2. Some amino acid residues are labeled with subscript numbers as reference points (e.g., AmCyan228 for the C terminus). The transmembrane strands (purple) of OmpA are numbered sequentially in parentheses. The N and C termini of AmCyan are highlighted in yellow. Membrane-spanning units and loops (blue) are based on the work of Francisco et al. (15) and Koebnik (25). The structure of the AmCyan protein was created with Cn3D software (available from the National Institutes of Health) based on crystallographic measurements from Wall et al. (52) and Yarbrough et al. (56).