Abstract
Introduction
The aim of this prospective study was to compare the visual functions of extended depth-of-focus intraocular lenses (EDOF IOLs) and monofocal IOLs in eyes with mild to moderate primary open-angle glaucoma (POAG).
Methods
Cataractous eyes with POAG controlled using medical treatments, no central visual field defects, and mean deviation (MD) values of −10 dB or better on the 30–2 test grid of the Swedish Interactive Threshold Algorithm standard program were included. Twenty-two eyes of 22 patients received EDOF IOLs (ZXR00V and ZXV150-375; J&J), whereas 24 eyes of 24 patients received monofocal IOLs (ZCB00V and ZCV150-375; J&J). MD values, corrected distance visual acuity (CDVA), and photopic contrast sensitivity were measured at 3 months after surgery. Noninferiority of CDVA and contrast sensitivity in eyes with EDOF IOLs to eyes with monofocal IOLs were examined.
Results
The postoperative mean MDs of eyes with EDOF and monofocal IOLs were −2.76 dB and −4.21 dB with no significant difference. The CDVA of eyes with EDOF IOLs was noninferior to that of eyes with monofocal IOLs (P = 0.02). There were no inferiority in contrast sensitivity at any spatial frequency (P < 0.001).
Conclusions
The visual function of EDOF IOLs in eyes with mild-to-moderate POAG was not inferior to that of monofocal IOLs.
Trial Registration
Registered in the Japan Registry for Clinical Research (identifier: jRCTs032200218).
Keywords: Contrast sensitivity, Extended depth-of-focus intraocular lens, Primary open-angle glaucoma, Visual function, Non-inferiority, Visual field
Key Summary Points
| Why carry out this study? |
| Presbyopia-correcting intraocular lenses (IOLs) have been implanted in normal cataractous eyes, while the use of bifocal IOLs has not been recommended for glaucomatous eyes. |
| Visions from far to intermediate distances can be provided with the use of extended depth-of-focus (EDOF) IOLs, and visual functions of eyes with EDOF IOLs are comparable to eyes with monofocal IOLs. |
| Thus, this prospective study aimed to compare the visual functions of EDOF and monofocal IOLs in eyes with mild-to-moderate primary open-angle glaucoma (POAG). |
| What was learned from the study? |
| Postoperative corrected distance visual acuity and contrast sensitivity of eyes with mild-to-moderate POAG and implanted EDOF lenses were not inferior to eyes with monofocal IOLs. |
| Comparable visual function with addition of better visual acuity from far to intermediate distances could be obtained with the use of EDOF IOLs, although careful patient selection and long-term evaluation for the progress of POAG are necessary. |
Introduction
Multifocal intraocular lenses (IOLs) are implanted in normal cataractous eyes to enable spectacle independence or reduce spectacle dependence postoperatively. However, implantation of such IOLs is generally restricted to eyes without ocular comorbidities because of the possible presence of photic phenomena and decreased contrast sensitivity in eyes with ocular diseases [1, 2]. Glaucoma is a common complication in cataractous eyes, and its progression is associated with a risk of decreased contrast sensitivity. The visual outcomes of refractive bifocal and diffractive trifocal IOLs in glaucomatous eyes have been studied; however, the numbers of eyes included in these studies were limited [3–5]. In general, implantation of bifocal IOLs in glaucomatous eyes is not recommended [1, 6, 7].
Diffractive extended depth-of-focus (EDOF) IOLs aid the achievement of optimal visual acuity at far to intermediate distances with the least photic phenomena. With the use of echelette grating optics, chromatic aberration is compensated and the least impact on visual function is achieved [8]. The postoperative contrast sensitivities of eyes with EDOF IOLs are comparable to those of eyes with monofocal IOLs [9]. In a recent case series, implantation of EDOF IOLs in 16 eyes of 10 patients with early-stage normal tension glaucoma (NTG) yielded satisfactory postoperative visual functions [10]. Although the comparability of EDOF IOLs to monofocal IOLs and the results of the above-mentioned report suggest that EDOF IOLs may be acceptable for certain glaucomatous eyes, it is important to examine the safety of EDOF IOLs in glaucomatous eyes. To the best of our knowledge, the visual functions of glaucomatous eyes with EDOF and monofocal IOLs have not yet been investigated and compared. Thus, the aim of this prospective study was to compare the visual functions of EDOF and monofocal IOLs in eyes with mild-to-moderate primary open-angle glaucoma (POAG).
Methods
Participants
This investigator-initiated prospective, open-label, comparative study was approved by a local certified review board and registered in the Japan Registry for Clinical Research (identifier: jRCTs032200218). The study was conducted in accordance with the tenets of the Declaration of Helsinki and the Clinical Trials Act of Japan (act no. 16, 2017). All patients provided written informed consent after receiving an explanation of the study protocol. Patients with POAG and cataract were recruited at the Tokyo Dental College Suidobashi Hospital (Tokyo, Japan) and Ryuundo Eye Clinic (Shiki, Japan).
The inclusion criteria were eyes with mild-to-moderate POAG, which was controlled using medical treatment for over 6 months, and diagnosed with no central visual field defect and a mean deviation (MD) value of −10 dB or better on the 30–2 test grid of the Swedish Interactive Threshold Algorithm (SITA) standard program (Humphrey Field Analyzer III; Carl Zeiss Meditec, Dublin, CA, USA). Stable and controlled intraocular pressure (IOP) was confirmed from the Goldmann applanation records from the previous two visits. MD values of −10 dB or better were determined by referring outcomes of the previous case of NTG eyes with EDOF IOLs [10]. Patients with eye diseases other than cataract (such as uncontrollable glaucoma, progressive diabetic retinopathy, uveitis, retinal detachment, iris neovascularization, or corneal degeneration), severe visual field defects (such as diffuse, central, or progressive defects), and risks of serious intraoperative complications (such as zonular rupture, posterior capsule rupture, vitreous prolapse, hyphema, or incomplete in-the-bag implantation) were excluded.
Sample Size
Based on the results of a previous retrospective study, the standard deviations (SDs) of contract sensitivity after implantation of EDOF IOLs ZXR00V (Johnson & Johnson Surgical Vision, Santa Ana, CA) were determined to be 0.23–0.24 at 12 and 18 cycles per degree (cpd) [11]. Hence, a sample size of 20 or more was necessary for examining non-inferiority of the study group to the control group with a margin of 0.20 logarithm contrast sensitivity (corresponding to over 1 step in the contrast sensitivity chart used), a significance level of 0.05, and detection power of 0.85 (package ‘TrialSize’ version 1. 3, R version 3.6.1). Considering 5% dropout in each group, the sample size was determined to be 25 eyes of 25 patients for each type of IOL.
Preoperative Examination and IOL Implantation
Preoperative examinations included IOP (Goldman applanation), axial length (IOLMaster 700, Carl Zeiss Meditec), automated perimetry (30–2 grid of the SITA program), and optical coherence tomography (CIRRUS, Carl Zeiss Meditec). To ensure the reliability of the automated perimetry results, the rates of fixation loss, false positive errors, and false negative errors were confirmed to be over 20%, 15%, and 33%, respectively. The MD values were obtained as well.
Implanted IOL was determined on the basis of the patient’s preference after explaining the characteristics of each type of IOL. As the postoperative visual acuities and the cost of surgery owing to the co-payment for the EDOF IOL were quite different, randomization was not performed. The implanted EDOF IOLs were ZXR00V and its toric models (ZXV150-375 and ZXW150-375), which are one-piece, violet light-blocking, aspheric, and hydrophobic acrylic lenses with continuous sharp optic edges on the posterior surface. The echelette optics on the anterior surface was designed to have a +1.75 D addition to provide an extended range of vision from 0.7 m and farther. The implanted monofocal IOLs were ZCB00V (Johnson & Johnson Surgical Vision) and its toric models (ZCV150-375 and ZCW150-375) with the same platform of the EDOF IOLs but without the echelette optics.
After removing the cataract using phacoemulsification and aspiration from a 2.4 mm temporal corneal incision, all IOLs were inserted into the capsular bag using specific injectors. For the implantation of EDOF IOLs, the powers were targeted for emmetropia, whereas the powers of monofocal IOLs were determined according to the patient’s preference.
Postoperative Examinations
Three months after surgery, automated perimetry, corrected distance visual acuity (CDVA), and contrast sensitivity were examined. Automated perimetry was performed in the same manner using 30–2 and 10–2 grids, and the MD and foveal threshold (FT) values were obtained because they are related to visual function. Visual acuity at 5 m was measured under photopic illumination using a Landolt ring chart. Manifest refraction spherical equivalent (MRSE) was also measured during the measurement of CDVA, and the value obtained was corrected to infinity by adding −0.20 D. Uncorrected distance visual acuity (UDCA) was measured as well. For eyes with EDOF IOLs, uncorrected and distance-corrected visual acuities (UCVA and DCVA, respectively) were measured at distances of 30 cm, 40 cm, 50 cm, and 1 m. The MRSE measured at 5 m was used for distance correction. Contrast sensitivity without glare was measured using CSV-1000 (Vector Vision, Fairfield, CT) under distance-corrected and photopic illumination (85 cd/m2).
Statistical Analysis
As IOP and some indexes of automated perimetry in POAG eyes change by cataract surgery [12], postoperative IOP, MD, and FT values were verified for properly comparing the visual functions of POAG eyes with EDOF and monofocal IOLs using an unpaired t-test.
The primary endpoint of this study was to examine the noninferiority of the CDVA and contrast sensitivity of eyes with EDOF IOLs to those with monofocal IOLs. Eyes with a CDVA of 20/40 (0.30 logMAR) or worse were excluded from the analysis to avoid the effects of diseases on visual function. For CDVA, noninferiority was examined with a margin of 0.15 logMAR, which corresponded to one to two steps in the chart used. The margin for contrast sensitivity at each spatial frequency was determined to be 0.20, which was between one and two steps in the chart used. P values were obtained using a t-test, which were used to analyze the difference in the margins. Statistical analysis was performed using SAS 9.4® software (version 9.4; SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Differences in UDVA and CVDA between eyes with EDOF and monofocal IOLs were examined using the Mann–Whitney U test. Differences in contrast sensitivity at 3, 6, 12, and 18 cpd, and MD and FT values were examined using a t-test.
Results
Of 50 eyes of 50 patients, 4 were excluded before and during surgery due to dropouts, lower MD preoperatively, and posterior capsule rupture. Consequently, 22 eyes received EDOF IOLs, whereas 24 eyes received monofocal IOLs. The mean IOP measured at two visits before enrollment was 13.4 (SD: 2.0) mmHg, and the preoperative mean IOP was 13.3 (SD: 2.0) mmHg; there was no significant change (P = 0.74, paired t-test). The demographic data of the patients are listed in Table 1. Although the differences between the preoperative IOPs of eyes with EDOF and monofocal IOLs were significant (P = 0.026), the mean difference of 1.3 mmHg was less than the acceptable measured error of 2 mmHg [13] and considered to be clinically neglectable. In the postoperative IOPs, there was no difference. Preoperative number of medications was higher in eyes with monofocal IOLs (P = 0.006). The mean axial length of eyes with EDOF IOLs was significantly longer by 1.5 mm.
Table 1.
Demographic data of the participants
| EDOF IOL | Monofocal IOL | P value* | |
|---|---|---|---|
| N, eye | 22 | 24 | |
| Age, year |
67.1 (9.6) [45–80] |
71.0 (6.4) [58–83] |
0.11 |
| Male/female | 10/12 | 11/13 | |
| Mean IOP, mmHg | |||
| Before enrollment |
12.9 (1.9) [9.5–16.0] |
14.0 (2.0) [11.0–16.5] |
0.070 |
| Preoperative |
12.6 (1.7) [10.0–15.7] |
13.9 (2.1) [11.0–18.3] |
0.026 |
|
Postoperative (3 months) |
12.6 (1.7) [9.3–15.7] |
12.8 (2.0) [8.0–16.7] |
0.65 |
| Number of medications |
1.3 (0.7) [0–3] |
2.0 (0.9) [1–4] |
0.006 |
| Axial length, mm |
26.3(2.0) [23.5–31.4] |
24.8(1.7) [22.3–28.5] |
0.012 |
| IOL models |
ZXR00V: 15 eyes ZXV150-375: 1 eye ZXW150-375: 6 eyes |
ZCB00V: 23 eyes ZCV150-375: 0 eye ZCW150-375: 1 eye |
Mean (SD) [range]
EDOF extended depth-of-focus, IOL intraocular lens, IOP intraocular pressure
*Unpaired t-test
The automated perimetry results preoperatively and 3 months after surgery are outlined in Table 2. While the mean preoperative MD of eyes with EDOF IOLs was better than that of eyes with monofocal IOLs (P = 0.030), there were no differences in the mean MD and FT values measured using the 30–2 and 10–2 grids after cataract surgery.
Table 2.
Automated perimetry results at preoperatively and 3 months after surgery
| EDOF IOL | Monofocal IOL | P value* | |
|---|---|---|---|
| Preoperatively | |||
| 30–2 grid | |||
| MD, dB |
−3.09 (2.65) [−8.96 to +1.32] |
−4.89 (2.82) [−9.79 to −0.95] |
0.030 |
| Postoperatively (3 months) | |||
| 30–2 grid | |||
| MD, dB |
−2.76 (2.30) [−6.79 to +2.02] |
−4.21 (3.29) [−12.53 to +0.48] |
0.09 |
| FT, dB |
34.5 (1.7) [30–37] |
35.2 (2.1) [31–39] |
0.28 |
| 10–2 grid | |||
| MD, dB |
−2.25 (2.23) [−6.01 to +1.97] |
−2.11 (2.89) [−10.76 to +1.87] |
0.85 |
| FT, dB |
34.5 (1.9) [30–38] |
35.3 (2.2) [31–40] |
0.21 |
Mean (SD) [range]
*Unpaired t-test
EDOF extended depth-of-focus, IOL intraocular lens, MD mean deviation, FT foveal threshold, dB decibel
The mean postoperative CDVAs of the included eyes are presented in Table 3. As the 95% confidence interval (95% CI) of the difference in CDVA (EDOF − monofocal) was between −0.14 and −0.04 logMAR, noninferiority of the CDVA of eyes with EDOF IOLs was confirmed (P = 0.020) with the margin of 0.15 logMAR. The cumulative UDVA and CDVA (20/x or better) measured at 3 months postoperatively are plotted in Fig. 1. All eyes with EDOF IOLs had a CDVA of 20/20 or better, whereas all eyes with monofocal IOLs had a CDVA of 20/25 or better.
Table 3.
Postoperative CDVA, MRSE, and contrast sensitivity results
| EDOF IOL | Monofocal IOL | P value | |
|---|---|---|---|
| CDVA, logMAR | −0.17 (0.10) | −0.08 (0.07) | 0.020a |
| MRSE, D | −0.39 (0.52) | −0.43 (1.08) | 0.87b |
| Contrast sensitivity | |||
| 3 cpd | 1.64 (0.17) | 1.67 (0.21) | < 0.001c |
| 6 cpd | 1.75 (0.19) | 1.86 (0.25) | < 0.001c |
| 12 cpd | 1.25 (0.24) | 1.41 (0.24) | < 0.001c |
| 18 cpd | 0.83 (0.30) | 0.92 (0.25) | < 0.001c |
Mean (SD)
EDOF extended depth-of-focus, IOL intraocular lens, CDVA corrected distance visual acuity, MRSE manifest refractive spherical equivalent, cpd cycles per degree
aNoninferiority test with a margin of 0.15 logMAR
bUnpaired t-test
cNoninferiority test with margins of 0.2
Fig. 1.
Cumulative distance visual acuity (20/x or better) of eyes with extended depth-of-focus (EDOF, left) and monofocal (right) intraocular lenses (IOLs) at 3 months postoperatively. UDVA uncorrected distance visual acuity; CDVA corrected distance visual acuity
The cumulative UCVA and DCVA (20/x or better) at 30 cm, 40 cm, 50 cm, and 1 m of eyes with EDOF IOLs are shown in Fig. 2. Preferred visual acuity was achieved at 1 m, and 20/25 or better was achieved in over 64% of the eyes at 50 cm. The mean UCVAs at 30 cm, 40 cm, 50 cm, and 1 m were 0.31, 0.16, 0.09, and 0.01 logMAR, respectively, whereas the DCVAs at 30 cm, 40 cm, 50 cm, and 1 m were 0.34, 0.19. 0.10, and −0.02 logMAR, respectively.
Fig. 2.
Cumulative uncorrected and distance-corrected distance visual acuities (20/x or better) of eyes with extended depth-of-focus intraocular lenses at 30 cm, 40 cm, 50 cm, and 1 m. UDVA uncorrected distance visual acuity, CDVA corrected distance visual acuity
Postoperative contrast sensitivity was measured in 22 eyes with EDOF IOLs and 23 eyes with monofocal IOLs. The mean contrast sensitivities at 3, 6, 12, and 18 cpd are presented in Table 3. As the 95% CIs of the differences in contrast sensitivity (EDOF − monofocal) at 3, 6,12, and 18 cpd were −0.14 to +0.09, −0.24 to +0.03, −0.32 to −0.02, and −0.25 to +0.09, respectively, noninferiority of contrast sensitivity in eyes with EDOF IOLs was confirmed (P < 0.0001) with the margin of 0.20. Figure 3 shows the box plots of the contrast sensitivities of eyes with EDOF (blue) and monofocal IOLs (green). There were no significant differences between the mean values of the eyes with EDOF IOLs and those of eyes with monofocal IOLs (P > 0.094; unpaired t-test with Holm’s multiple adjustments). There were eyes below the normal range at high spatial frequencies; contrast sensitivities of eight and six eyes with EDOF and monofocal IOLs, respectively, were less than 0.70 at 18 cpd. However, there was no difference of the occurrences between IOLs (P = 0.46, chi-squared test).
Fig. 3.
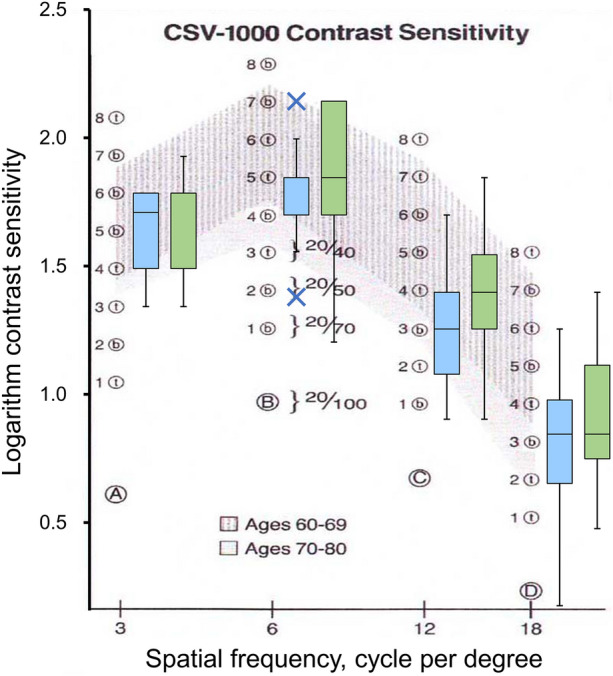
Box plots of the postoperative contrast sensitivities of eyes with extended depth-of-focus (blue) and monofocal (green) intraocular lenses
Discussion
This study demonstrated that, after implantation of diffractive EDOF IOLs in eyes with mild-to-moderate POAG and no central visual field defect, the CDVA and contrast sensitivities were not inferior to those of eyes with monofocal IOLs. To the best of our knowledge, this is the first study in which postoperative visual functions, such as contrast sensitivity and visual field, of cataractous eyes with POAG implanted with EDOF IOLs were compared with those of such eyes implanted with monofocal IOLs. Results of optical bench tests and previous clinical studies have indicated that the optical properties and contrast sensitivities of eyes with EDOF IOLs did not differ from those of eyes with monofocal IOLs [9, 14]. Regarding the influence of EDOF IOLs on visual field tests, the MD and FT of normal eyes with EDOF IOLs do not differ from those of normal eyes with monofocal IOLs [15, 16]. In addition, a previous NTG case series on eyes with the same EDOF IOLs as those used in the present study reported similar visual functions [10]. The results of the present study and those of previous findings supported the safety of EDOF IOLs for certain eyes with mild-to-moderate POAG 3 months postoperatively.
Regarding contrast sensitivity, there was no statistically significant difference between the two IOLs. However, some eyes showed lower than the norm at 18 cpd. A couple of factors, such as implantation of a multifocal IOL and progression of POAG, would reduce contrast sensitivity at high spatial frequencies. Reduced contrast sensitivity at 18 cpd is commonly observed in eyes with diffractive multifocal IOLs, owing to the superimposition of blurred images from the added powers [2, 17]. In the present study, the add power of the EDOF IOL was 1.75 D, which is lower than those of the multifocal IOLs used in previous studies. Thus, the influence of the add on contrast sensitivity in the present study would be small, but not negligible. Visual field sensitivity is another factor that affects contrast sensitivity. Diffractive bifocal and trifocal IOLs decrease MD values [15, 18], thereby increasing the risk of reduced contrast sensitivity [19]. In the eye with EDOF IOL that showed the lowest contrast sensitivity at 18 cpd, the MD in the 30–2 grid was −6.14 dB. We speculated that lower visual field sensitivity would be affected; however, further investigation is required to confirm this.
This study had limitations. First, only photopic contrast sensitivity was evaluated. It should be noted that glaucomatous eyes have an increased risk for reduced mesopic contrast sensitivity [19]. Liu et al. addressed that the scotopic contrast sensitivity of eyes with EDOF IOLs is worse than that of eyes with monofocal IOLs [17]. In future studies, mesopic and scotopic contrast sensitivity is needed. Second, only eyes with relatively well-controlled POAG (MD values of −10 dB or better, no central defect, and stable under medication) were included in this study. As reduction in contrast sensitivity is associated with central sensitivity of the retina [19], it is anticipated that eyes with more severe POAG would show significant reduction in visual function. Implantation of EDOF IOLs in eyes with severe POAG would be restricted for ethical and safety reasons. However, it would be valuable to comprehensively study how individual visual field parameters affect contrast sensitivity in eyes with mild-to-moderate POAG. Lastly, long-term visual function following the implantation of EDOF IOLs in eyes with POAG is still unknown. Aptel et al. reported that the 1-year progression rate of MD in eyes with treated POAG was −0.32 dB in the early stage (MD > −6 dB) and −0.52 dB in the moderate stage (MD −6 to −12 dB) [20]. Based on these results, it is possible that even eyes with MD values of −10 dB or better may experience a decrease in contrast sensitivity, especially in the high-frequency range, if their MD values deteriorate over time. We believe that, for eyes with POAG, long-term analysis of visual function in those with EDOF IOLs is crucial.
Conclusion
A prospective comparative study demonstrated that the visual function of EDOF IOLs in eyes with mild-to-moderate POAG was not inferior to that of monofocal IOLs. While careful patient selection and long-term investigation are necessary, it is anticipated that monofocal IOL-comparable visual function with better visual acuity from far to intermediate distances could be obtained with the use of EDOF IOLs in mild to moderate POAG eyes.
Acknowledgements
We thank the participants of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization and Funding acquisition: Hiroko Bissen-Miyajima, Keiichiro Minami; Investigation: Hiroko Bissen-Miyajima, Yuka Ota, Yoko Taira; Project administration: Hiroko Bissen-Miyajima; Methodology: Yuka Ota, Yoko Taira; Formal analysis: Ryo Takemura; Validation and Writing–original draft: Keiichiro Minami; Writing–review, editing, and approval: all authors.
Funding
This investigator-initiated trial study was supported by Johnson & Johnson Surgical Vision. No funding or sponsorship was received for the publication of this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Hiroko Bissen-Miyajima reports grant, consultant, and speaker honorarium from Alcon and Johnson & Johnson Surgical Vision, consultant from BVI and Zeiss, speaker honorarium from BVI and Hoya. Yuka Ota reports speaker honorarium from Johnson & Johnson Surgical Vision. Keiichiro Minami reports Patent pending from Tomey. Yoko Taira and Ryo Takemura declare that they have no competing interests.
Ethical Approval
This investigator-initiated study was approved by the Certified Review Board, Hattori Clinic and registered in the Japan Registry for Clinical Research (identifier: jRCTs032200218). The study was conducted in accordance with the tenets of the Declaration of Helsinki and the Clinical Trials Act of Japan (Act No. 16, 2017).
References
- 1.Braga-Mele R, Chang D, Dewey S, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313–322. doi: 10.1016/j.jcrs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Schallhorn JM, Pantanelli SM, Lin CC, et al. Multifocal and accommodating intraocular lenses for the treatment of presbyopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128:1469–1482. doi: 10.1016/j.ophtha.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi M, Kinoshita S. Implantation of refractive multifocal intraocular lens with a surface-embedded near section for cataract eyes complicated with a coexisting ocular pathology. Eye (Lond) 2015;29:649–655. doi: 10.1038/eye.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamath GG, Prasad S, Danson A, Phillips RP. Visual outcome with the array multifocal intraocular lens in patients with concurrent eye disease. J Cataract Refract Surg. 2000;26:576–581. doi: 10.1016/S0886-3350(99)00457-5. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez- Sánchez C, Rementería-Capelo LA, Puerto B, et al. Visual function and patient satisfaction with multifocal intraocular lenses in patients with glaucoma and dry age-related macular degeneration. J Ophthalmol. 2021 doi: 10.1155/2021/9935983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teichman JC, Ahmed II. Intraocular lens choices for patients with glaucoma. Curr Opin Ophthalmol. 2010;21(2):135–143. doi: 10.1097/ICU.0b013e3283365154. [DOI] [PubMed] [Google Scholar]
- 7.Ichhpujani P, Bhartiya S, Sharma A. Premium IOLs in glaucoma. J Curr Glaucoma Pract. 2013;7:54–57. doi: 10.5005/jp-journals-10008-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millán MS, Vega F. Extended depth of focus intraocular lens: chromatic performance. Biomed Opt Express. 2017;8(9):4294–4309. doi: 10.1364/BOE.8.004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedrotti E, Bruni E, Bonacci E, Badalamenti R, Mastropasqua R, Marchini G. Comparative analysis of the clinical outcomes with a monofocal and an extended range of vision intraocular lens. J Refract Surg. 2016;32:436–442. doi: 10.3928/1081597X-20160428-06. [DOI] [PubMed] [Google Scholar]
- 10.Bissen-Miyajima H, Ota Y, Yuki K, Minami K. Implantation of diffractive extended depth-of-focus intraocular lenses in normal tension glaucoma eyes: a case series. Am J Ophthalmol Case Rep. 2022;29:101792. doi: 10.1016/j.ajoc.2022.101792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirasawa M, Ota Y, Ooki S, Minami K, Bissen-Miyajima H. Visual function after implantation of extended depth of focus intraocular lenses using Echelette design. Atarashii Ganka. 2019;36:161–164. [Google Scholar]
- 12.Lee JW, Morales E, Yu F, et al. Effect of cataract extraction on the visual field decay rate in patients with glaucoma. JAMA Ophthalmol. 2014;132:1296–1302. doi: 10.1001/jamaophthalmol.2014.2326. [DOI] [PubMed] [Google Scholar]
- 13.Sandhu SS, Chattopadhyay S, Amariotakis GA, Skarmoutsos F, Birch MK, Ray-Chaudhuri N. The accuracy of continued clinical use of applanation tonometers with known calibration errors. Ophthalmology. 2009;116:9–13. doi: 10.1016/j.ophtha.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Choi M, Xu Z, Zhao Z, Alexander E, Liu Y. Optical bench performance of a novel trifocal intraocular lens compared with a multifocal intraocular lens. Clin Ophthalmol. 2016;10:1031–1038. doi: 10.2147/OPTH.S106646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Mori Y, Nejima R, Minami K, Miyata K. Influence of implantations of extended depth-of-focus on standard automated perimetry. Sci Rep. 2020;10:20153. doi: 10.1038/s41598-020-77214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi M, Yamashiro C, Yoshimoto T, et al. Influence of extended depth of focus intraocular lenses on visual field sensitivity. PLoS ONE. 2020;15:e0237728. doi: 10.1371/journal.pone.0237728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Dong Y, Wang Y. Efficacy and safety of extended depth of focus intraocular lenses in cataract surgery: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19:198. doi: 10.1186/s12886-019-1204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farid M, Chak G, Garg S, Steinert RF. Reduction in mean deviation values in automated perimetry in eyes with multifocal compared to monofocal intraocular lens implants. Am J Ophthalmol. 2014;158:227–231. doi: 10.1016/j.ajo.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Lahav K, Levkovitch-Verbin H, Belkin M, Glovinsky Y, Polat U. Reduced mesopic and photopic foveal contrast sensitivity in glaucoma. Arch Ophthalmol. 2011;129:16–22. doi: 10.1001/archophthalmol.2010.332. [DOI] [PubMed] [Google Scholar]
- 20.Aptel F, Aryal-Charles N, Giraud JM, et al. Progression of visual field in patients with primary open-angle glaucoma: ProgF study 1. Acta Ophthalmol. 2015;93:e615–e620. doi: 10.1111/aos.12788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




