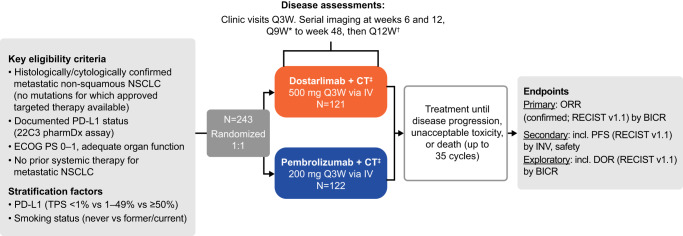
Fig. 1. Study design.
*Or more frequently, if clinically indicated. †Imaging will continue to be performed until discontinuation of study treatment due to disease progression with clinical instability, start of subsequent anticancer treatment, withdrawal of informed consent, or death, whichever comes first. ‡Up to 35 cycles pemetrexed with either cisplatin or carboplatin for the first four cycles. BICR blinded independent central review, CT chemotherapy, DOR duration of response, ECOG PS Eastern Cooperative Oncology Group performance status, INV investigator assessment, IV intravenous, NSCLC non-small cell lung cancer, ORR overall response rate, PD-L1 programmed death ligand 1, PFS progression-free survival, QxW every x weeks, RECIST v1.1 Response Evaluation Criteria in Solid Tumors version 1.1, TPS tumor proportion score.