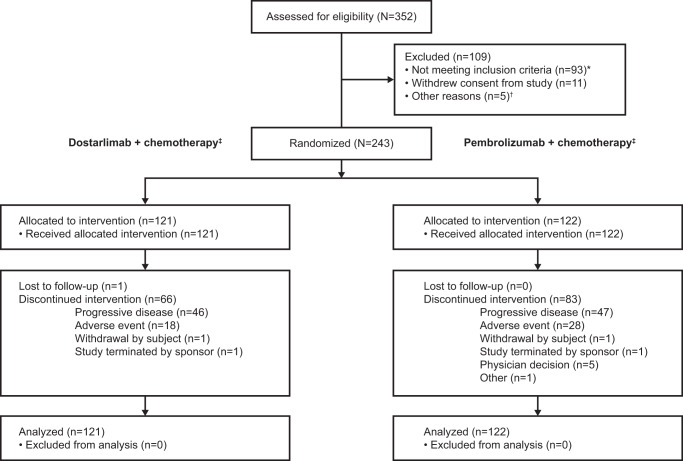
Fig. 2. CONSORT flow diagram.
*Most common reasons were patients not meeting the inclusion criteria for confirmed metastatic non-squamous NSCLC with documented absence of genomic aberration with available approved target therapy (n = 24) and for documented PD-L1 status by the 22C3 pharmDx assay (n = 22). †Other reasons included death (n = 4) and lost to follow-up (n = 1). ‡Pemetrexed plus platinum-based therapy (either cisplatin or carboplatin). CONSORT Consolidated Standards of Reporting Trials, NSCLC non-small cell lung cancer, PD-L1 programmed death ligand 1.