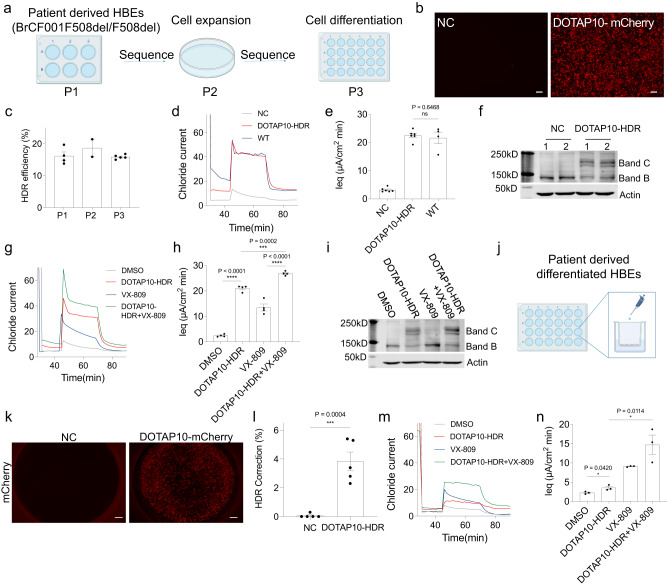
Fig. 5. DOTAP LNPs successfully corrected CFTR mutation and restored CFTR function in patient-derived human bronchial epithelial (HBE) cells with F508del mutation.
a Undifferentiated HBE cells with F508del mutation (passage 1, P1) were treated with DOTAP10-HDR for 4 days before cell expansion (passage 2, P2). One week after cell expansion, cells were established on transwell inserts for cell differentiation (passage 3, P3). The untreated group was used as negative control (NC) and HBE cells from a healthy donor expressing wild-type (WT) CFTR was used as positive control. b DOTAP10 LNPs encapsulating mCherry mRNA (DOTAP10-mCherry) were efficiently internalized into P1 HBE cells, showing bright mCherry fluorescence. Scale bar: 500 μm. c, The HDR correction efficiency maintained at as high as 16% after a single DOTAP10-HDR treatment, as evaluated in P1, P2, and P3 HBE cells. DOTAP10-HDR treatment successfully restored CFTR activity, compared to WT CFTR activity (d), as confirmed by area under the curve (AUC) analysis (e). Data are shown as mean ± s.e.m. (n = 4–6 biologically independent samples). f Expression of CFTR protein was detected after treatment of DOTAP10-HDR (1, 2 means repeats). Co-treatment of DOTAP10-HDR and VX-809 enhanced Cl- transport and improved CFTR function (g), with corresponding AUC values (h). There was a significant difference between VX-809 and DOTAP10-HDR + VX-809 groups with a P value < 0.0001. Data are shown as mean ± s.e.m. (n = 4 biologically independent samples). i Increased expression of CFTR protein was detected after co-treatment of DOTAP10-HDR + VX-809. j, DOTAP10-HDR were directly added onto the surface of differentiated HBE cells. k DOTAP10-mCherry was efficiently internalized into fully differentiated HBE cells, resulting in bright mCherry fluorescence. Scale bar: 500 μm. l DOTAP10-HDR successfully corrected F508del mutation in differentiated HBE cells. Data are shown as mean ± s.e.m. (n = 5 biologically independent samples). m Co-treatment of DOTAP10-HDR and VX-809 significantly enhanced Cl- transport and improved CFTR function in differentiated HBE cells, as confirmed with corresponding AUC values (P = 0.0114) (n). Data are shown as mean ± s.e.m. (n = 3 biologically independent samples). Two-tailed unpaired t-tests were used to determine the significance (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Data of (b, k) were repeated three times independently with similar results.