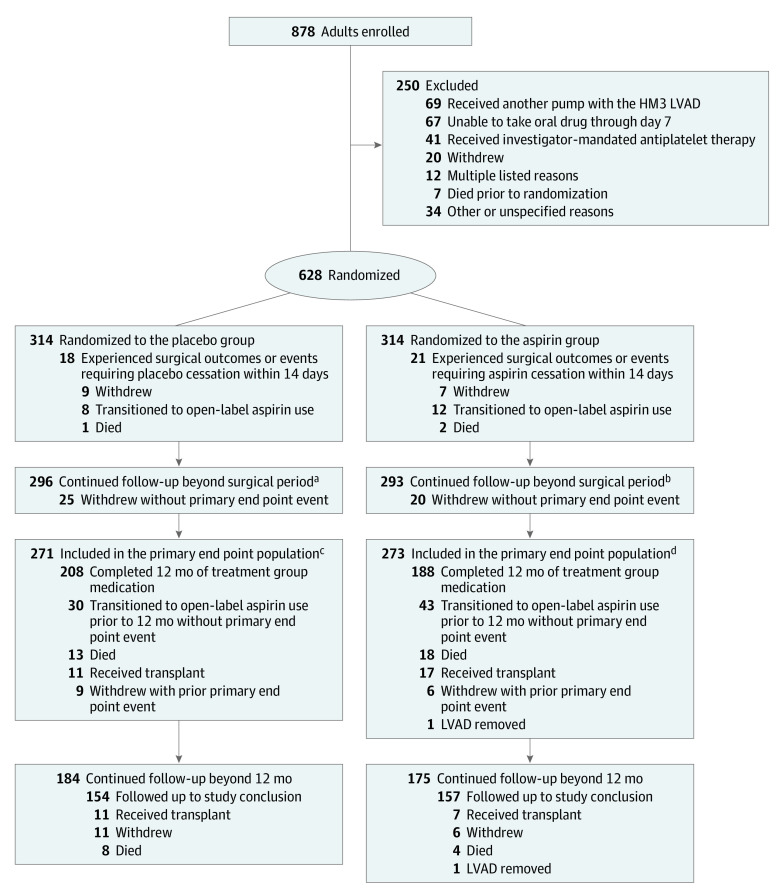
Figure 1. Flow of Patients in the Randomized Trial of Placebo Compared to Aspirin With a Vitamin K Antagonist in Patients With a Fully Magnetically Levitated Left Ventricular Assist Device.
Patients were followed up until 12 months for the evaluation of the primary end point in the primary end point set. Patients who continued receiving the treatment group medication (placebo or aspirin) at 12 months were eligible for continued follow-up beyond 12 months. Reasons for patient withdrawal are shown in eTable 1 in Supplement 2. Details on patient transition to open-label aspirin use can be found in eTables 2 and 3 in Supplement 2.
aMedian (IQR) of 14.2 (9.0-21.4) months.
bMedian (IQR) of 14.3 (7.2-20.6) months.
cConcluded follow-up at 12 months (n = 53).
dConcluded follow-up at 12 months (n = 55).