Figure 2. Estimates of the Probability of Death or Nonsurgical Major Hemocompatibility-Related Adverse Events and Nonsurgical Bleeding Events.
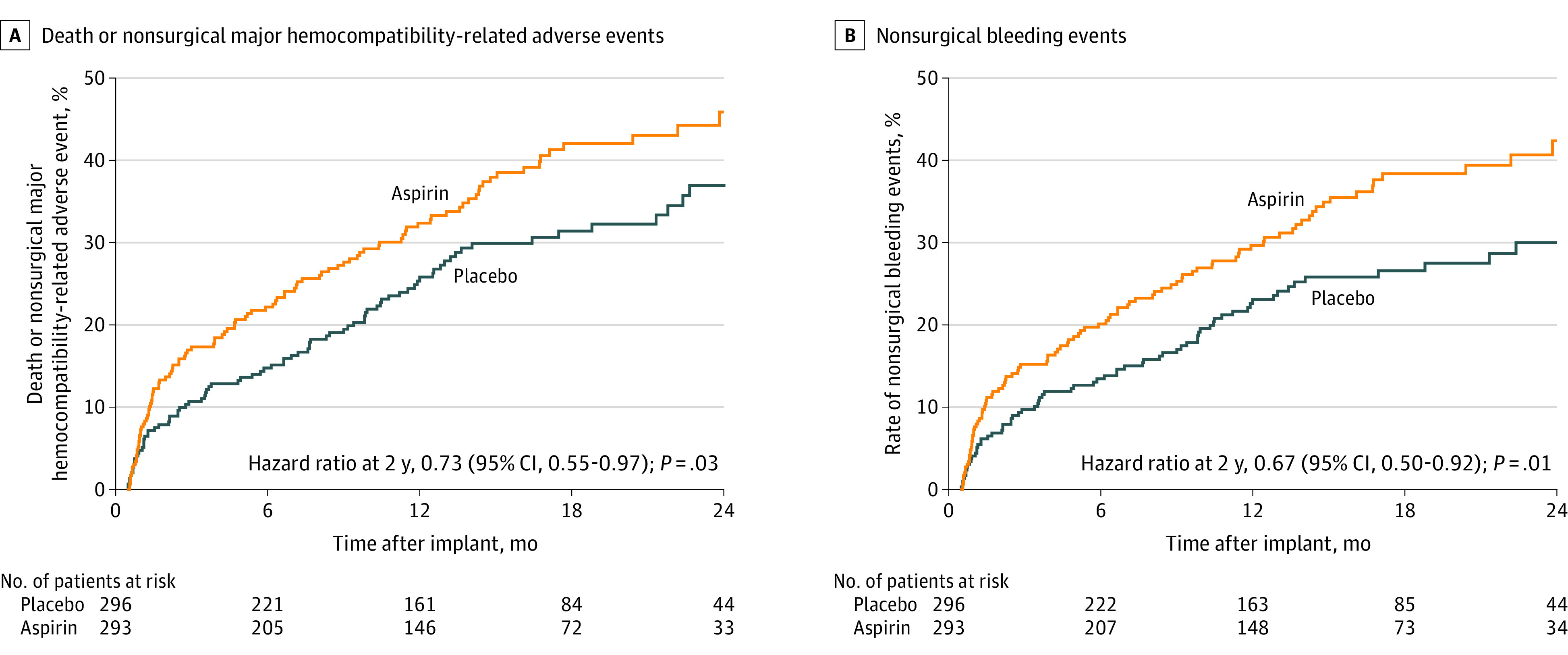
The probability of death or nonsurgical major hemocompatibility-related adverse event reflects the failure of the primary end point. In this 24-month time-to-event analysis, patients in the placebo group experienced decreased probability of primary end point failure at 12 months (25.8 for placebo vs 32.4 for aspirin) and 24 months (36.9 for placebo vs 45.9 for aspirin; log-rank P = .03). Decreased primary end point failure was driven by decreased nonsurgical bleeding at 12 months (23.1 for placebo vs 29.7 for aspirin) and at 24 months (30.0 for placebo vs 42.4 for aspirin; log-rank P = .01). Hazard ratios were calculated by Cox proportional hazard modeling with treatment group as the only effect. Adjusted hazards models are provided in eFigure 10 in Supplement 2. Median (IQR) follow-up was 14.2 (9.0-21.4) months in the placebo group and 14.3 (7.2-20.6) months in the aspirin group.
