Figure 3. Principal Secondary End Point Details and Safety End Points .
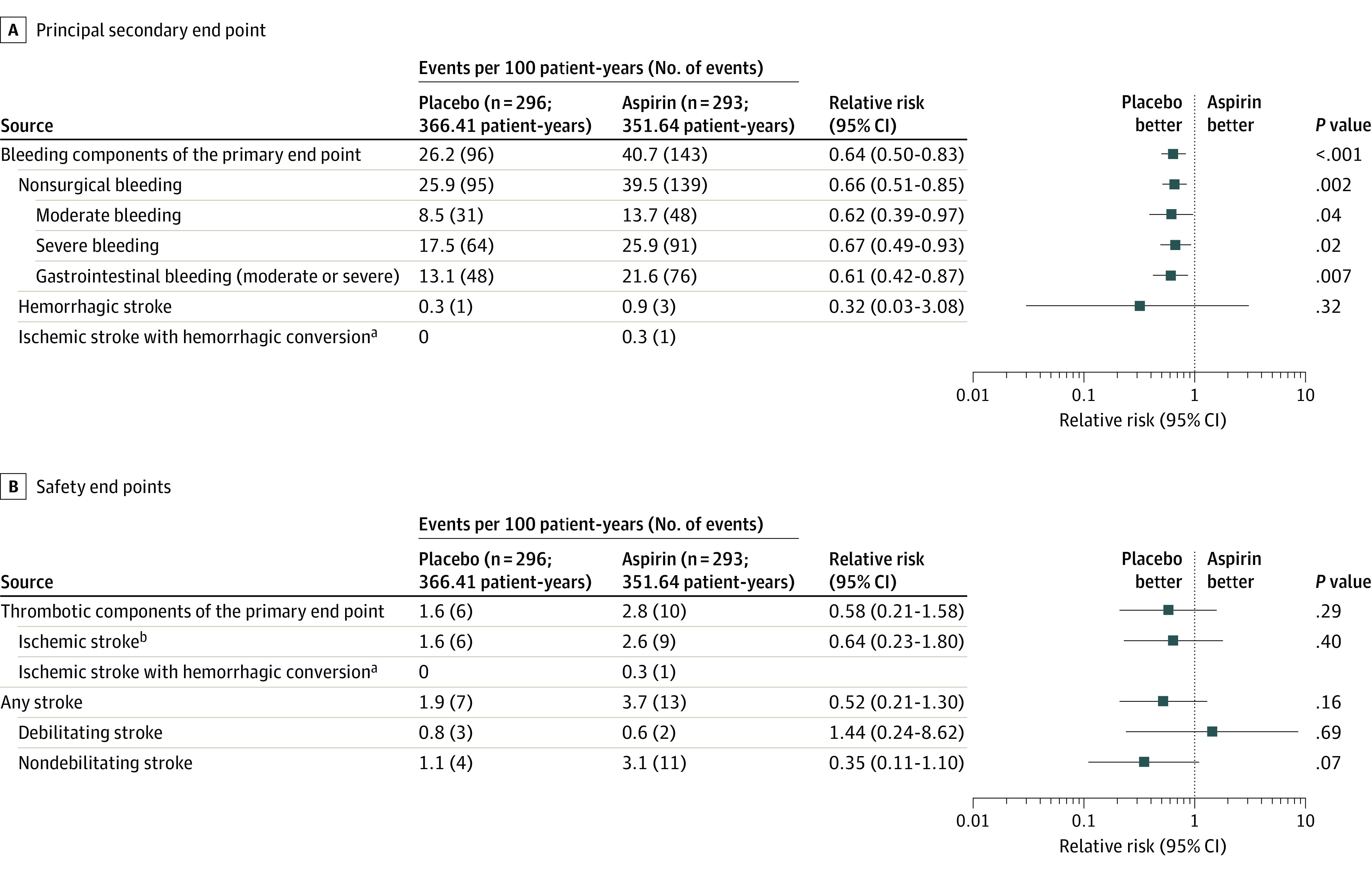
With the avoidance of aspirin, the relative risk of bleeding (the principal secondary end point, including recurrent bleeding) was reduced with no change in safety end points. Bleeding event reduction is observed in both moderate and severe bleeding categories as well as gastrointestinal bleeding. Bleeding events included those that occur while hospitalized or that led to hospitalization or urgent care and required medical intervention. Details on classification as moderate or severe are available in Supplement 2. Events are shown as events per 100 patient-years (to account for first and recurrent events). The relative risk is depicted as a forest plot with 95% CIs and P values. Time-to-event analyses of stroke (eFigure 5 in Supplement 2), overall survival (eFigure 6 in Supplement 2), thrombotic components (eFigure 11 in Supplement 2), and bleeding components of the primary end point (eFigure 12 in Supplement 2) are provided in the Supplement 2. Adjusted hazard models of major adverse events are shown in eFigure 10 in Supplement 2.
aAn ischemic stroke with hemorrhagic conversion event is counted within both the composite “bleeding components of the primary end point” and “thrombotic components of the primary end point.”
bExcludes ischemic stroke with hemorrhagic conversion.
