Abstract
Effective tumor treatment depends on optimizing drug penetration and accumulation in tumor tissue while minimizing systemic toxicity. Nanomedicine has emerged as a key solution that addresses the rapid clearance of free drugs, but achieving deep drug penetration into solid tumors remains elusive. This review discusses various strategies to enhance drug penetration, including manipulation of the tumor microenvironment, exploitation of both external and internal stimuli, pioneering nanocarrier surface engineering, and development of innovative tactics for active tumor penetration. One outstanding strategy is organelle-affinitive transfer, which exploits the unique properties of specific tumor cell organelles and heralds a potentially transformative approach to active transcellular transfer for deep tumor penetration. Rigorous models are essential to evaluate the efficacy of these strategies. The patient-derived xenograft (PDX) model is gaining traction as a bridge between laboratory discovery and clinical application. However, the journey from bench to bedside for nanomedicines is fraught with challenges. Future efforts should prioritize deepening our understanding of nanoparticle-tumor interactions, re-evaluating the EPR effect, and exploring novel nanoparticle transport mechanisms.
Keywords: Nanomedicine, Tumor penetration, Penetration models, Tumor microenvironment, Organelle-affinitive transfer
Graphical abstract
Highlights
-
•
Models for assessing drug penetration into tumors are compared.
-
•
Strategies for structural transformation of nanomedicine in the tumor environment are comprehensively elaborated.
-
•
Strategies to overcome tumor barriers for deep penetration are provided.
-
•
Active tumor penetration is introduced.
-
•
The potential and hurdles of nanomedicine in tumor therapies are concluded.
1. Introduction
Cancer remains a pervasive health issue plaguing the modern society. Conventional treatment methods, including radiation therapy, chemotherapy, targeted therapy, and immunotherapy [1,2], have been used to combat cancer, however, either low response rates or severe adverse effects demand new therapeutic modalities for cancer treatment. A recently-developed nano-scale formulation with a nanostructure, nanomedicine, offers advantages including increased bioavailability of drugs [3,4], prolonged circulation and retention [5], and controlled drug release patterns. Despite these advantages [6], the therapeutic effect of nanomedicine is limited due to a complicated tumor microenvironment (TME). The milieu surrounding tumor cells includes tumor stem cells, stromal cells, microvessels, lymphatic vessels, fibroblasts, immune cells, and extracellular matrix (ECM) [7]. Multiple barriers in the milieu hinder drug delivery from nanomedicine into solid tumors to achieve an effective therapeutic dose.
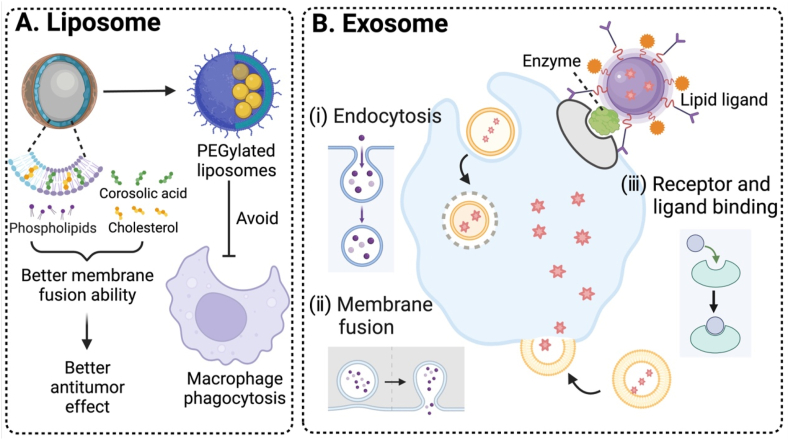
To address these barriers, one promising approach is surface modification of nanomedicine [8]. Surface modification typically involves altering chemical compositions or structural properties of nanomedicine and/or incorporating targeting ligands or functional molecules [[9], [10], [11], [12]], resulting in changes in chemical/physical/biological properties such as surface charges and enhanced targetability to mitigate non-specific distribution. This is critical because unmodified nanomedicine is often engulfed by the reticuloendothelial system (RES) and it often accumulates in organs such as livers and spleens [13]. Surface modification can contribute to reduced phagocytosis and enhanced passive tumor accumulation via the enhanced permeability and retention (EPR) effect. In addition, modification with active targeting ligands can achieve precise targeting of tumor cells. To enhance tumor penetration, surface engineering techniques of nanomedicine using liposomes, cell-secreted exosomes, or cell-penetrating peptides have been developed [[14], [15], [16], [17], [18]]. These entities can be tailored for surface modification based on specific characteristics of the TME to realize deep tumor penetration.
Considering the dense and complicated TME, targeting the ECM, a major component of the TME, is emerging as a strategic approach to modulate the TME [[19], [20], [21]]. The ECM plays a pivotal role in tumor progression, making it a prime target to facilitate the specific entry of nanomedicines into the tumor interior. One direction in nanoparticle strategies is to loosen and degrade the dense ECM using various proteolytic enzymes. Futhermore, deep penetration of nanomedicines can also be achieved actively. This shift from passive to active processes, facilitated by mechanisms such as the intercellular transport, cation-mediated active transcytosis, and other attempts to explore novel active transcellular transfer, is gaining traction and emerging as an important trend. This could potentially reshape the design of nanomedicine and overcome tumor penetration barriers for tumor therapy.
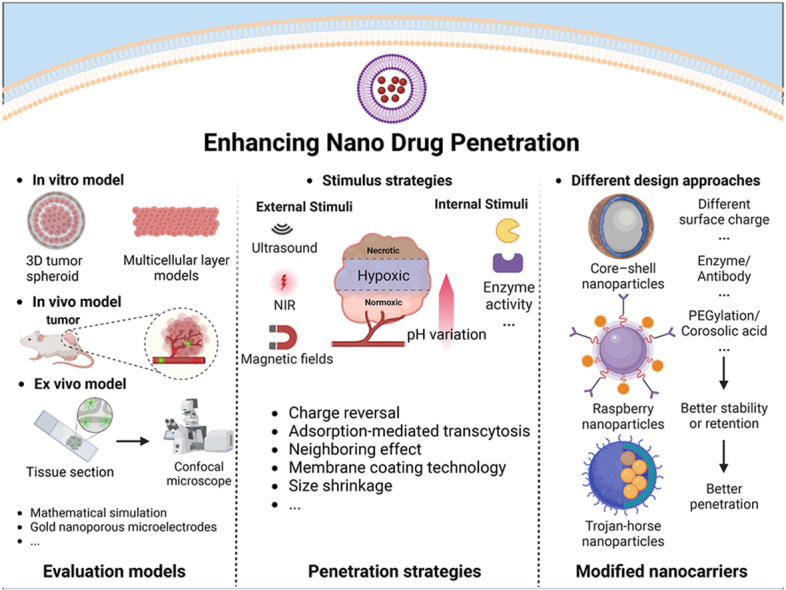
These strategies for developing multi-functional, targeted nanomedicine to enhance tumor penetration are essentially relied on the effectiveness of overcoming multiple biological barriers in the complex TME. Those biological barriers in the TME have been mimicked via in vitro 3D tumor spheroids and multiple-layer cell structures, ex-vivo tissues collected from animal models, or in-vivo tumor tissues after implanting tumors into the animals. The degree of drug penetration through nanomedicine could have a significant discrepancy among these experimental models, and these experimental outcomes may not be clinically translatable. In this review, the experimental models are critically reviewed for evaluating the penetration ability of nanomedicine. The start-of-the-art strategies for preparing nanomedicine to facilitate penetration into solid tumors are elaborated. Surface engineering of nanomedicine for enabling its interaction with tumor cell membranes for facilitating drug penetration is discussed. Perspectives in these strategies are offered.
2. Models for evaluation of the degree of tumor penetration
The rate at which drug molecules penetrate through a membrane or overcome a barrier is fundamental for drug permeability, particularly when evaluating the level of intestinal absorption and drug bioavailability. Under physiological conditions, drug molecules need to overcome a variety of barriers and avoid clearance before reaching the tumor tissue, which is one of the recognized hallmarks of solid tumors. To address the challenge of insufficient drug doses in the tumor tissue, strategies for rational design of drug carriers and effective application of a drug delivery system have been explored to enhance drug penetration into solid tumors. Success of these strategies pertains to accurate assessment of drug permeability in the preliminary test, including how to evaluate the degree of drug penetration accurately and effectively, how to establish the test conditions mimicking actual malignant lesions in the body, and how to select a corresponding system to predict the permeability effect. This section presents a few innovative methods to achieve drug penetration evaluation. Fig. 1 graphically represents three commonly employed models of assessing tumor infiltration.
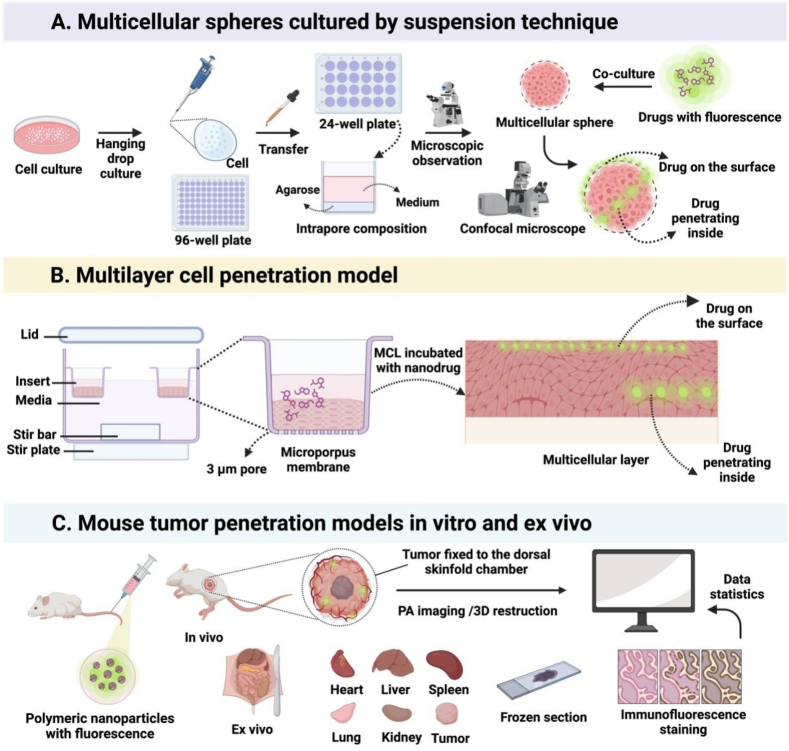
Fig. 1.
Illustration of the procedures for 3 different tumor penetration evaluation models. (A) Cultivation of multicellular spheroids via the hanging droplet method, followed by drug incubation and observation of drug penetration into the depth of multicellular spheroids under confocal microscopy. (B) A multilayer cell penetration model by introducing drugs into the cell culture media. (C) Evaluation of drug permeability via in vivo and ex vivo imaging. (Fig. 1 was created with BioRender.com.).
2.1. In vitro models
2.1.1. Multicellular layers
A multicellular layer (MCL) model to simulate the tumor environment in vitro was first proposed by Wilson and his colleagues [22]. Fig. 2A graphically illustrates two commonly used MCL models. The initial purpose of using a MCL is to study the extravascular pharmacokinetics and determine whether the resistance of solid tumors to chemotherapy originated from limited tissue permeability of anticancer drugs [23]. Gradually, it is utilized to real-time assess the anti-proliferative effect of medications and the impact of specific protein expression on the tissue permeability [24,25]. Experimental cells in the exponential growth phase are seeded into collagen-coated microporous Teflon membranes before they are placed into the culture plate for roughly one week to create the model. The formed MCL has a symmetric, planar structure and it possesses a few characteristics of solid tumors, such as comparable ECM properties and short gaps similar to those between epithelial cells [23,26]. Drugs to be tested are added to one side of the MCL for incubation, and they are detected on the other side of the MCL at various time intervals. The penetration of paclitaxel (PTX) or 5-fluorouracil (5-FU) into human colorectal cancer cells (DLD-1 and HT-29) was assessed using the MCL model [27]. Although the location of nanomedicine in the body and the level of its accumulation in tumor tissues remain mysterious after they depart blood arteries, the MCL model has been employed to examine the penetration and distribution of nanomedicine [28] (Fig. 2B and C).
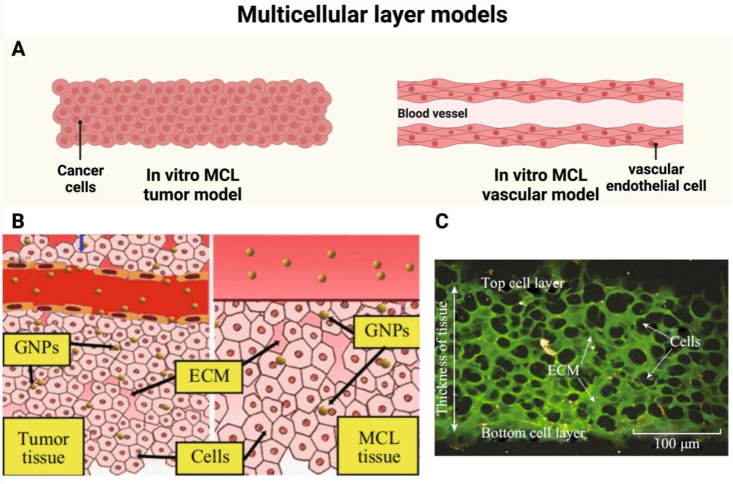
Fig. 2.
Application of multicellular layer models for assessing tumor penetration. (A) Schematic representation of in vitro multicellular layer models for tumor and vascular studies. (B) Illustration of the multicellular layer model in the culture medium containing GNPs compared to the solid tumor tissue. (C) Cross-sectional view of the MCF-7 tissue stained with eosin for the extracellular matrix (green). Unstained areas correspond to cells. Scale bar: 100 m. Note: GNPs, gold nanoparticles. (Fig. 2A was created with BioRender.com. Fig. 2B and C were reprinted with permission from Ref. [28]. Copyright 2014 Springer Nature.).
The advantage of the MCL in the evaluation of tumor penetration is that the amount of drugs can be accurately measured after they penetrate through a multicellular-layered tissue, which offers similar properties such as cell adhesion and an equivalent compact structure compared to solid tumors. This model could more accurately capture the ecology of the tumor microenvironment than 2D models such as single-layer cells, due to stacking of multiple layers of tumor cells. However, the in vitro MCL model can not display a complete characteristic map of solid tumors, including blood vessels within the tissue, the ECM, cell proliferation behaviors, nutrient concentration gradients, gene expression patterns, cell-cell interactions and cell-ECM interactions.
In addition, vascular osmosis is important in construction of the MCL model. To mimic a vascular osmosis model, human umbilical vein endothelial cells (HUEVC) are frequently utilized. A transwell kit is utilized for the osmosis model. Vascular endothelial-like cells are cultured in the upper chamber of the transwell kit, which has a porous membrane, while the selected tumor cells are incubated in the lower chamber of the unit. The impact on the vascular osmosis is often evaluated by changes in the fluorescence intensity of tumor cells [[29], [30], [31]].
2.1.2. Multicellular spheroids
The traditional cell culture model is a 2D cell model by culturing monolayer cells on the surface of cell culture vessels and it has been used to simulate human development and disease progression. However, due to simple physical contacts between cells and lack of organizational structures and complexities [32], the 2D cell model can not mimic the human body tissue. With advances in stem cell biology and organ regeneration, state-of-the-art 3D cell culture models can more accurately mimic the cellular microenvironment, cellular interactions, and physiological and biochemical reactions than single-layer 2D cell models [33]. Since the twenty-first century, this technology has gained popularity in many fields and it has been routinely used for the evaluation of cytotoxic T cell attacks, drug resistance, and tumor cell invasion, as well as revelation of their corresponding mechanisms [[34], [35], [36], [37]].
3D cell culture models can be built with the help of a scaffold or without a scaffold (Fig. 3A). The mostly commonly used scaffold is hydrogels [38]. To mimic the extracellular matrix for better protection of 3D cell spheres, Tayalia et al. created an affordable semi-synthetic cryogel matrix system [39] derived from polyethylene glycol diacrylate (PEGDA) and gelatin methacryloyl (GELMA). This matrix system facilitated spheroid growth to establish a hierarchical structure, thus mimicking in vivo ECM components and offering complex structural supports (Fig. 3B). Since it was constructed from expandable materials, the model could be used to simulate complex extracellular environments. Kim et al. developed an in vitro 3D cancer model [38] by integrating multicellular spheroids within a composite ECM hydrogel to demonstrate its potential in examining phenotypic shifts in pancreatic cancer during tumor advancement (Fig. 3C). It has been demonstrated that crosslinked polymers in an aqueous solution can solidify to form hydrogels after a change in the temperature [40], allowing cells to embed in the hydrogels, which can more closely mimic the cellular milieu in the body. In addition, support materials derived from human or other sources can be used to mimic extracellular environments. The self-aggregation ability of cells, which enables cells to form microspheres and maintain a three-dimensional configuration during the culture process, is the foundation for scaffold-free 3D culture models with fine structures. Scaffold-free 3D models are often created by the use of ultralow attachment solid supports or the hang drop technique [41] (Fig. 3D). The ultra-low adsorption plate has a neutral charge or hydrophilic proteins, which prevents cell attachment onto the wall of the plate, thus forming 3D aggregates. The disadvantage of the plate lies in its short-term stability. A straightforward technique for 3D cell culture models is developed from the hang drop cell culture system. It is usually operated via a hang drop plate or a 96-well plate lid [42]. The cells in the hang drop settle under the action of gravity to form cell microspheres.
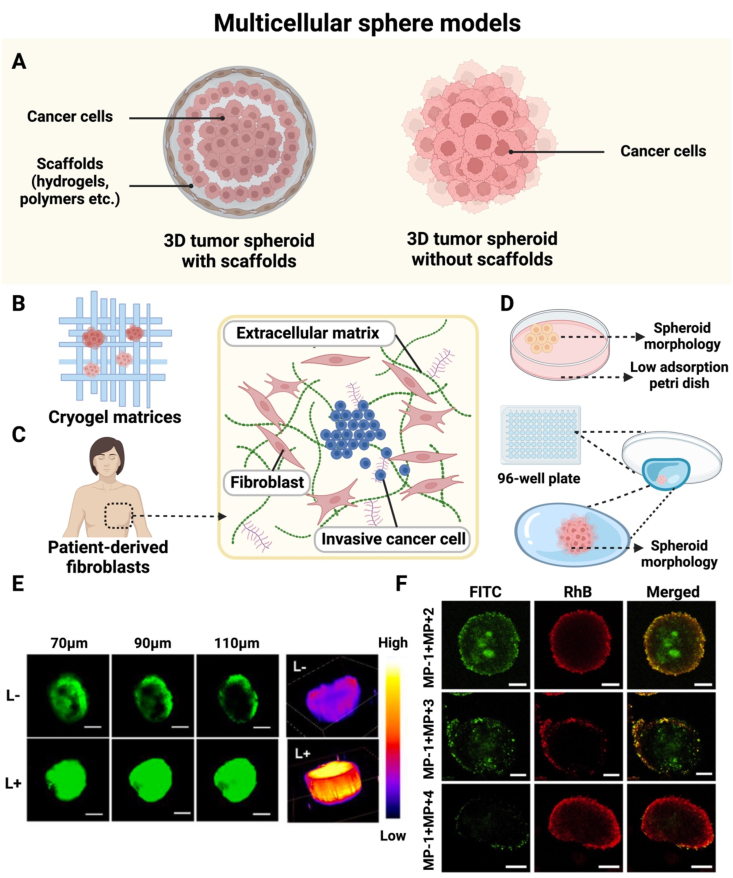
Fig. 3.
Application of 3D tumor cell spheroids for assessing tumor penetration. (A) Schematic illustration of fabricating 3D tumor cell spheroids with or without scaffolds. (B) Schematic of the morphology of cancer cell spheroids on cryogel matrices. (C) Multicellular spheroids of invasive cancer cells produced in the environment created by human-derived stromal cells. (D) A spheroid formed by cancer cells cultivated on low-adhesion culture dishes and 96-well plate lids. (E) Tumor penetration of ID@M − N, a dual-nanotransformer for size and charge, in MSCs observed by Z-stack CLSM (scale bar: 100 μm) and 3D fluorescence analysis of CLSM images at a depth of 150 μm. (F) CLSM images of optical slices through the centers of SH-SY5Y-labeled MSCs incubated with 3 different combinations for 24 h. MP-1 was FITC-labeled. (Fig. 3A–D were created with BioRender.com. Fig. 3E was reprinted with permission from Ref. [30]. Fig. 3F was reprinted with permission from Ref. [44]. Copyright 2016 Elsevier.).
The most often utilized in vitro model for examining the penetration of therapeutic nanoparticles or drug delivery systems into tumors is a 3D tumor spheroid, also known as a multicellular cancer spheroid (MCS) obtained from scaffold-free 3D cell culture models. This experimental approach, for instance, was utilized to compare the level of tumor penetration between RLPA-NPs and LPA-NPs [43]. Fluorescein isocyanate (FITC) can be specifically chosen for co-incubation in the formation of multicellular spheroids (MCSs) [30] (Fig. 3E), enabling confocal observation of MCSs in a 3D dimension [44,45] (Fig. 3F). From the MCS images of confocal laser scanning microscopy (CLSM), the intensity of the fluorescence in MCSs can be assessed. For example, the fluorescence intensity of MCSs treated with drugs can be measured from the periphery to the center of MCSs for statistical analysis of the level of drug penetration [46]. To examine the variations in the penetration of different drugs [47], the cell spheres can be disassembled to single cells and these single cells are then subjected to flow cytometry analysis to generate quantitative information on fluorescence intensity. The in vitro osmosis model is derived from the MCS model [48], but it has the option of including a small amount of collagen to mimic the ECM and act as a barrier to prevent the diffusion of nanoparticles. Additionally, stroma-rich MCSs were developed [49] to mimic solid tumors in the human body, and they could be used to accurately depict the permeability of nanomedicine to a certain extent.
Compared to the MCL, fabrication of MCSs has a shorter period, and cytokines and adhesion factors can be added to mimic a solid tumor microenvironment. As a result, MCSs have been widely used as an in vitro 3D model. Although the suspension technique is relatively easy to create MCSs, it is challenging to control the size of the cell spheres. Furthermore, some of tumor cell lines do not have the capacity to form spheroids. Although the above in vitro 3D tumor penetration models are multicellular to mimic solid tumors to some extent and they can be employed in evaluating the penetration level of different drug formulations, both models cannot accurately mimic vascular perfusion and other biological characteristics of solid tumors from a practical standpoint. While multicellular spheroids and multilayered cells often serve as in vitro models, they face a few challenges. In particular, they lack the vasculature, which plays a critical role in tumor progression and metastasis in vivo. Additionally, these in vitro models can not completely map the complexity of an in vivo tumor environment. Furthermore, current fabrication methods for these in-vitro models can vary significantly, for example, the degree of densification of cell spheroids could be dramatically different between tumor models, thus therapeutic results of nanomedicine basing on these in-vitro models may not be used for apple-to-apple comparison. Efforts should be made into standardization of the preparation procedure for these in-vitro models and optimization of these models for completely mapping the tumor microenvironment.
2.2. In vivo model
The penetration of nanomedicines or small-molecular drugs can also be observed using an in vivo model. The term “in vivo models” in this article refers to an animal as a whole body or its organs/tissues of interest for in-vivo analytical imaging studies and organs/tissues harvested from an in-vivo animal model for ex vivo imaging studies. The observation of the permeability in solid tumors is generally executed on a tumor-bearing mouse model (Fig. 4A). Imaging technologies play a central role in the observation in the animal model. There are several widely used molecular imaging techniques, including visible light imaging, tomography imaging, magnetic resonance imaging (MRI) [50,51] (Fig. 4B), and ultrasound imaging (US). However, a low resolution is typical issue of these techniques and they do not allow for dynamic monitoring. The penetration depth of solid tumors for these commonly used in vivo methods are elaborated below.
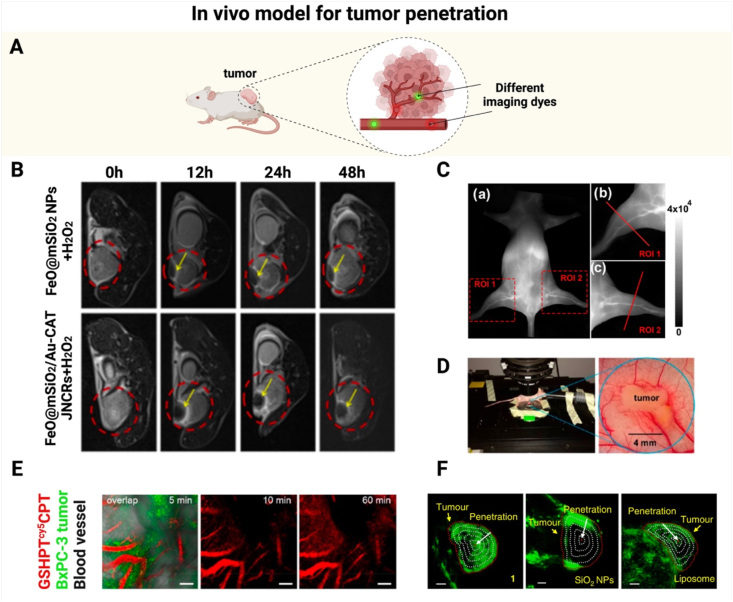
Fig. 4.
In vivo models for assessing tumor penetration. (A) Schematic illustration for applying different dyes to visualize blood vessels in a tumor-bearing mouse. (B) In vivo MRI scan images of tumors. (C) (a) NIR-II imaging of mouse blood vessels at 10 min after intravenous administration of a SQ1 nanoprobe. (b, c) Zoomed-in NIR-II images for two specified regions of interest (ROI 1 and ROI 2) in the hind limbs. (D) CLSM imaging of a dorsal skinfold chamber model. (E) In vivo monitoring of temporal distribution of GSHPTcy5CPT in tumor blood vessels. (F) 3D reconstruction PA images of tumor bearing mice injected with molecule 1, SiO2 nanoparticles and liposome labeled by Cy. The tumor tissue was divided into five parts with identical radius intervals. Scale bar: 2 mm. Note: SQ: squaraine dye; NIR-II: near-infrared light II; CLSM: confocal laser scanning microscopy; GSHPTcy5CPT: a GGT-triggered charge-reversal dendrimer-CPT conjugate marked with cy5. (Fig. 4A was created with BioRender.com. Fig. 4B was reprinted with permission from Ref. [51]. Copyright 2023 American Chemical Society. Fig. 4C was reprinted with permission from Ref. [53]. Copyright 2020 American Chemical Society. Fig. 4D and E were reprinted with permission from Ref. [55]. Copyright 2020 American Chemical Society. Fig. 4F was reprinted with permission from Ref. [59]. Copyright 2019 Springer Nature.).
Traditional optical imaging is only applicable at a shallow depth of less than 1.0 mm, thus it has limited application in solid tumor imaging. Near infrared two-region imaging (1000–1700 nm) is a new optical imaging technique. Compared to other imaging techniques, it has a higher imaging resolution and a greater penetration depth. Near-infrared is frequently used for real-time monitoring of small blood vessels with a penetration depth of about 30 mm. Amphiphilic polymers are typically used in this application because of their ability to self-assemble [52]. Yao et al. successfully constructed a fibronectin-targeting SQ1 nanoprobe via the donor-acceptor-donor (D-A-D) engineering methodology [53], and the nanoprobe had an outstanding performance in near-infrared light II (NIR-II) angiography and tumor imaging (Fig. 4C). To study the degree of penetration, different dye molecules are available to label blood vessels. For example, at 2 h after intravenous injection of a fluorescently labeled drug into a tumor-bearing mouse, Dylight-488 conjugated tomato lectin is intravenously injected to stain blood vessels and the mouse is sacrificed at 5 min post-injection. Confocal laser scanning is then performed after collection of the tumor tissue. As a result, the degree of deep penetration into solid tumors can be assessed for different small molecular drugs [54]. Through the intrinsic fluorescence of nano-carriers or a fluorescent probe equipped on nano-carriers, the depth of nano-carrier progression into tumors can be real-time monitored.
Huang and his team applied a whole-body optical imaging technique on a tumor model [31]. The skin of the model mice was cut along the midline of the abdomen and the blood vessels were examined under a microscope using a caliper [55] (Fig. 4D). The vascular peripherals could be readily observed in the photographs. After liposomes were labeled with Cy5, their penetration in the solid tumors could be observed. In a similar manner, real-time observation of Cy5-labeled vectors during US cavitation-induced tumor penetration was realized using CLSM and a US apparatus [29]. In an in vivo model, Shen and his team did not stain blood vessels. Instead, they used Cy5.5 or Cy5 to label vectors and then computed the mean total fluorescence intensity of vectors in the solid tumors for pertinent analysis [55,56] (Fig. 4E).
Photoacoustic imaging (PAI), a painless imaging technology, uses a laser to produce an ultrasound wave that helps view the contents inside the body. After giving animals 1 % sodium pentobarbital anesthesia, Yin and his team opened arcs around tumors without harming blood arteries [57]. The PAI signals were captured via a Vevo LAZR imaging system. Real-time observation of drug tumor penetration was achieved. For therapeutic agents or nanocarriers without fluorescent signal, fluorescent labeling of agents/nanocarriers is usually conducted to allow for penetration observations under CLSM. For instance, Cy5 can be incorporated to therapeutic agents to real-time record the fluorescence signal of Cy5 in the tumor to map biodistribution of these agents [58]. PAI was used for continuous tomography and 3D image reconstruction then carried out (Fig. 4F). In this process [59], a tumor-selective cascade activatable self-detained system was used and marked with an additional cyanine dye. PAI was also applied to measure the tumor permeability of nanomaterials (nanoclusters) with inherent fluorescence in an A549 tumor-bearing mouse model, and intravital real-time CLSM imaging was conducted to detect the fluorescence signal of the nanomaterials. Semi-quantitative analysis was carried out to determine the fluorescence intensity of the nanomaterials within the tumor delineated by PAI signal [60].
The above approaches have been employed for measuring osmosis in vivo with optical markers. They are comparable to in vitro techniques for assessing penetration. In a study on the permeability effect of liposomes, the established tumor-bearing nude mice were first injected with FITC-labeled liposomes, and the tumor tissues were harvested after the mice were sacrificed. The tissues were fixed with 4 % paraformaldehyde [61], and the blood vessels were stained with DAPI and labeled with an anti-CD31 antibody. The tissues were then examined via CLSM. Similar strategies have been used to evaluate the intratumoral penetration of nanomedicine [62,63]. Tumor sections can also be used to measure osmosis ex vivo. After tumor tissues are removed and cut into 10.0 m pieces [64], they can be examined under a fluorescence microscope 24 h after the mice receive the drug. Imaging technology has also been used to assess the degree of drug penetration ex vivo. Unlike in vivo imaging, the pre-processing time for ex vivo imaging is fixed. There are still some disparities between groups [65], such as inconsistent doses of drug administration, variations in the tumor-formation processes in the mice, and various treatment time periods. Overall, after establishing an in vivo animal model, there are discrepancies two individual images obtained from in vivo or ex vivo imaging, and these discrepancies may be reduced through the use of multiple experimental replicates within the same group. In vivo imaging involves anesthetizing an animal and cutting its skin for observation, which may have an effect on the healthy status of the animal, therefore, the imaging duration may be significantly reduced and long-term observation of the animal cannot be realized. The ex vivo tissue sectioning process may quench fluorescent-carrying substances or biological characteristics of sections, and the experimental operation should be carefully designed and strictly controlled to minimize the impact from the sectioning process.
2.3. Mechanistic simulations and tools for assessment of penetration effects
In addition to the commonly used animal models and in vitro cytosphere models mentioned earlier, a number of mechanistic simulations or detectors have been explored for in vivo animal experiments and they are of great interest for assessing the therapeutic effect of nanomedicine. Fukumura and his team proposed a multi-stage approach [66] to constructing gelatin-core nanoparticles (QDGelNPs) at a size of about 100 nm, and 10 nm quantum dots (QDs) in the core of the nanoparticles with fluorescence signal were encapsulated in the gelatin. QDGelNPs served as a nanomedicine simulation model to mimic the process of drug penetration to reach deeper layers of the tumor tissue. Additionally, 5-kDa polyethylene glycol (PEG) was attached to the nanoparticle surface, allowing for long-term blood circulation. A HT-1080 tumor model which has high MMP-2 activity was selected for the simulation nanoparticle. QDGelNPs were MMP-2-responsive to achieve the release of 10 nm QDs upon arrival at the tumor site. Tumor penetration parameters were acquired by in vivo imaging. This simulation system with a surface coating has a changeable shape, and it enables charge reversal, the tumor penetration parameters can be tuned according to the system design requirements. In practical application, we may be able to use this system to predict the drug release rate as well as the penetration degree of nanomedicine in a solid tumor tissue.
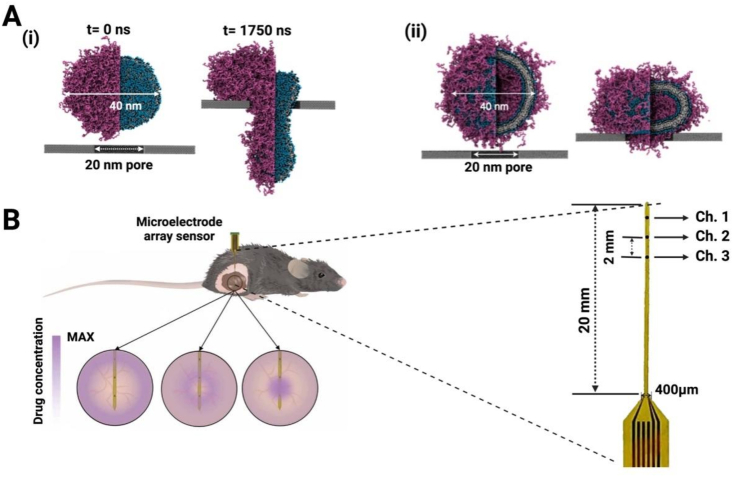
A stiff pore with a diameter of 20 nm was created in a coarse-grained molecular dynamics simulation model to account for the permeability difference between lipid nanodiscs (LNDs) and liposomes [67] (Fig. 5A). The results revealed that a flaking structure of LNDs composed of PEGylated lipids and high-melting-point phospholipids may perforate and spread more rapidly than traditional liposomes. Morphological modifications to facilitate the perforation could be beneficial for deep distribution of STING agonists. Deep penetration could also be achieved by nanoparticle deformation from layer by layer (LbL) NPs. Stiff-LbL NPs and Compliant LbL NPs were prepared with different levels of stiffness. The impact of the carrier deformability on tumor penetration was assessed from the degree of filter blockage and the presence of pre-labeled NPs in the filtrate using ultrafast centrifugation [68]. Therefore, the degree of deformation of nanoparticles could be optimized via in vitro simulations before they can applied to in vivo tumor penetration.
Fig. 5.
Special experimental methods to study tumor penetration. (A) Coarse-grained simulation snapshots of the perforation behavior of (i) liposomes and (ii) nanodiscs. (B) Gold nanoporous microelectrodes implanted in mice to monitor the concentration of chemotherapy drugs at different time points. Detailed images of the enlarged sensor are also provided (Fig. 5A was reprinted with permission from Ref. [67]. Copyright 2022 Spinger Nature. Fig. 5B was reprinted with permission from Ref. [69]. Copyright 2022 AAAS).
A technique was recently developed to real-time monitor pharmacokinetics in live animal tumor tissues and assess tumor penetration. A microelectrode array sensor was used to track the concentrations of doxorubicin (DOX). The electrodes were vertically aligned in the center of the tumor tissue for in vivo monitoring of DOX when the mouse was under anesthesia. The entire apparatus [69], which resembles a broom, could continuously monitor the drug level in different tumor areas (Fig. 5B). The drug concentration in tumor tissues after surgical excision was detected in addition to in vivo intratumoral tests, confirming this device was able to detect the drug level at different depths of the tumor tissue from each independent channel of the electrode. Thus, this device could provide a novel means of evaluation of drug penetration in solid tumors by monitoring the drug concentration at various periods post-injection and at various depths of tumors. However, the flexible sensor has a specific requirement for the size of the tumor, which prevents its applicability to other animal tumor models. The position for the sensor insertion in tumor-bearing mice may vary with individual operators, which could become an issue for the application of the tool.
In addition, the degree of antibody drug penetration into tumors was evaluated as early as 10 years ago. In order to theoretically explain the findings and offer a context for drug absorption design [70], mathematical analysis of systematic clearance and the impediment before the antibody reached the tumor parenchyma was performed. A mathematical mechanistic model was built for simulating physiological tumor drug delivery [71], and the penetration rates of nanoparticles at a size in a 1–250 nm range were predicted after anti-angiogenic drugs were applied to change the pore size distribution. The predictions suggest that vascular normalization combined with anti-angiogenic therapy can improve the effective delivery of small-sized therapeutic drugs, but not large-sized drugs. Similarly, in the development of tumor drug screening models for breast cancer, linear regression analysis was performed to reveal a quantitative structure-activity relationship that can help predict drug penetration into tumors [72], when a three-dimensional multi-cellular sphere model to simulate ECM deposition was selected for drug screening. The infiltration models could be combined with in vitro or in vivo models to predict the infiltration degree of drugs, offering great insights into rational design of nanomedicine to improve drug penetration in solid tumors. Taken together, it will become essential for the development of models and tools for assessing tumor penetration, while the simulation of a real tumor environment, the maintenance of the healthy state of tumor-bearing mice, and the development of scientific assessment methods require a more innovative, integrated and comprehensive approach.
2.4. PDX models
Discrepancies between the preclinical and clinical trials results of nanomedicine is are a big obstacle in its development, and the establishment of animal models to mimic a real tumor microenvironment is crucial for biological evaluation of nanomedicine. Many tumor models have been developed for biological evaluation of nanomedicine, including subcutaneous or in situ tumor models based on tumor cell lines, human-derived tumor transplantation models, or genetically engineered mice. Homotransplantation models, also known as cell-line-derived xenograft (CDX) tumor models, constitute the majority of animal models utilized in the in vivo and ex vivo studies discussed in the previous section. Unfortunately, CDX models cannot completely address the complexity of patient tumor tissues [73]. Tumor heterogeneity is credited to significant differences in tumor proliferation and invasion [74], and the model to address tumor heterogeneity is critical for assessing nanomedicine for tumor diagnosis and treatment. To pursue an animal model that mimics a complex and realistic physiological or pathological environment in patient [75], patient-derived xenograft (PDX) models have been developed, and they have a number of advantages. The PDX model is built by transplanting tumor tissues from patients into critically immunodeficient mice. Those immunodeficient mice have a low rejection rate for heterologous tissues, which pronouncedly improves the success rate of construcing a PDX model. The tumor tissues grow inside the mice to form the first-generation transplanted tumors. The third-generation and above mice can be subjected to drug therapy. The PDX model can preserve the parental tumor growth microenvironment to a greater extent, and they are more conducive to retention of parental tumor traits and maintain tumor heterogeneity compared to other animal models [76].
The PDX model has become popular for assessing the anti-tumor effect of nanomedicine. Post-surgical resection tissues from patients diagnosed with muscle invasive bladder cancer were transplanted subcutaneously through the skin to the NSG mice. The tumors were then transplanted into BALB/c nude mice after the tumors in NSG mice grew to a size of 500 mm3, and the PDX model after three generations was then used for in vivo evaluation of Poly(OEGMA)-PTX@Ce6 (NPs@Ce6) that consisted of a photosensitizer chlorin e6 and a histone B-sensitive polymer PTX [77]. After H&E staining of the transplanted tumors in the mice, this PDX model inherited histopathological features of the parental tumors. Subsequent examination of biodistribution and in vivo antitumor effects of NPs@Ce6 after its systemic administration showed that NPs@Ce6 demonstrated a strong inhibitory effect on tumor volume expansion after short-long-term irradiation, and tumors in 2 out of 5 mice were completely ablated. In another example [78], tumor tissues of a prostate cancer patient were collected and planted the tumor blocks subcutaneously in the right hind limb to establish a PDX model to establish cRGD-MDTX, cRGD-directed docetaxel micelles with a small size and high stability. Within a 30-day cycle after administration, cRGD-MDTX displayed remarkable inhibition of tumor growth. They also established an A549 in situ graft tumor model as well as a PC3 subcutaneous graft tumor model, confirming that cRGD-MDTX had comparable anti-tumor activity in vivo, and a density of cRGD-MDTX at 4 % significantly blocked tumor progression.
Apart from conventional CDX models and emerging PDX models [79], humanized mice have been constructed to equip an animal model with a human immune environment, thus human immune response to the administrated nanomedicine could be properly assessed in the animal model. The development of humanized mice is complementary to the PDX model. These humanized mice could carry functional human genes, cells or tissues. Humanized mice are typically created through human stem cell transplantation [80], which are differentiated into humanized organs or tissues to determine the feasibility of the model. Humanization in the mice can also be accomplished by implantation of umbilical cord blood, bone marrow, and adult mobilized HSCs [81,82]. The xenograft mouse tumor model has a human-mimicking immune system and human tumor tissues, thus it could accurately capture the human immune response [83], and can be used for clinical therapeutic regimen development studies [[84], [85], [86], [87], [88]] on nanomedicine for immunotherapy or vaccines and combine therapy with immunotherapy [89].
Despite the advantages of the PDX model including capturing the genetic complexity of the parental tumor, it has a few challenges before it becomes a workhorse in preclinical studies. There are many factors for the success of PDX xenografts in mice, including the tolerance of a mouse host, individual differences between mice, and the location of the xenograft [90]. The mortality rate of the mice for the PDX model is often very high before establishing a mature PDX model for a specific tumor. In addition, the PDX model often cannot sustain tumor growth in vivo. Although conditional reprogramming (CR) technology can be applied to maintain the stability of the PDX model without affecting basic biological properties [91], the CR technology is currently confined to an in vitro model, thus it is not able to mimic interactions of various substances in an actual tumor environment [92].
PDX models are often preferential for studies on small-sized nanomedicine penetration. A redox-responsive copolymer-POEG-co-PVDGEM (PGEM) was used to co-deliver gemcitabine (GEM) and PTX [93], and this small-sized GEM-conjugated polymer-based nanomedicine was examined in a pre-partially knocked-down PDX model. The nanomedicine displayed effective accumulation within the tumor in the PDX model, and the degree of its penetration in the tumor tissues was assessed by staining of tumor sections. During the application of the PDX model, we can consider its combination with a CDX model or the CR technology in a complementary way to achieve specific requirements for preclinical evaluation of nanomedicine.
3. Strategies to improve drug penetration into solid tumors
The following sections will discuss how to create effective and efficient nanoscale drug delivery systems for nanomedicine and improve current nanomedicine platforms from the perspective of penetration. Strategies on manipulating the size/charge of nanoparticles or nanosystems in response to unique features of the TME, overcoming physical/chemical/biological barriers of solid tumors, and remodeling of the TME are elaborated.
3.1. The ECM of the TME
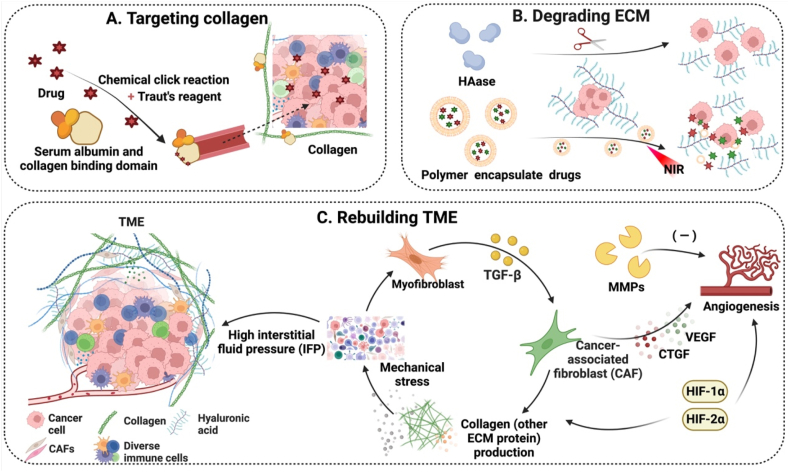
TME is an extremely complex structure and it plays an essential role in drug permeability into solid tumors [[94], [95], [96]]. Drug penetration becomes more critical for larger tumors [97,98]. There are three major constitutional components of the TME: matrix components, cellular components, and soluble factors [99]. It is well known that the TME has a high interstitial fluid pressure (IFP), which is derived from various internal mechanical pressures because the ECM, including collagen and hyaluronic acid, becomes denser and denser as the tumor develops. Cancer-associated fibroblasts (CAFs) in the TME not only promote the formation of the ECM, but also have an indirect effect on the IFP. CAFs also induce microenvironmental homeostasis in some types of gastrointestinal cancer. The formation and origin of CAFs remains mysterious, but one hypothesis is that they are produced because of epithelial to mesenchymal transition (EMT). CAFs may originate from trans-differentiation of surrounding cells mediated by transforming growth factor (TGF-) [100]. TGF- not only acts on fibroblasts, but also increases the myofibroblast population. It also participates in differentiation of different surrounding fibroblasts to CAFs, therefore, promoting ECM production and stimulating angiogenesis by secreting connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), and other cytokines. The increasingly dense microenvironment in turn imposes a barrier for oxygen diffusion or transfer; and oxygen is excessively consumed by proliferative tumor cells, thus creating an oxygen-poor or hypoxia microenvironment. Tumor cells are able to gradually adapt themselves to the hypoxic condition, due to hypoxic-inducing factors (HIFs). In the hypoxia TME, the protein stability of HIFs is greatly enhanced.
To overcome the resistance for drug delivery into the dense network of the ECM including collagen and hyaluronic acid, targeted substances can be coated to the surface of nanomedicine. The ECM can also be degraded by the action of enzymes in nanomedicine. When the dense ECM becomes “loose”, the resistance of drug delivery in these networks could be greatly diminished. To modulate the entire microenvironment, special soluble factors that are important for the formation of the microenvironment could be elegantly manipulated, including CAFs, TGF- that promotes the formation of CAFs, and HIFs that induce tumor cells to become adapatable to the hypoxia environment. Strategies for harnessing the above-mentioned ECM characteristics for nanomedicine development are elaborated in this section. These strategies for modulating the tumor microenvironment are shown in Fig. 6.
Fig. 6.
Schematic illustration of the mechanisms of modulating the tumor microenvironment to achieve deep penetration. (A) Targeting collagen in the microenvironment by albumin conjugates to enhance deep penetration. (B) Degradation of the ECM to facilitate transport of drugs encapsulated in polymers. (C) Molecules involved in remodeling the tumor microenvironment to promote drug penetration. Note: ECM, extracellular matrix; HAase, hyaluronidase; TME, tumor microenvironment; IFP, high interstitial fluid pressure; CAF, cancer-associated fibroblast; TGF-, transforming growth factor ; MMPs, matrix metalloproteinases; HIFs, hypoxic-inducing factors; CTGF, connective tissue growth factor; VEGF, vascular endothelial growth factor. (Fig. 6 was created with BioRender.com.).
3.1.1. Targeting collagen
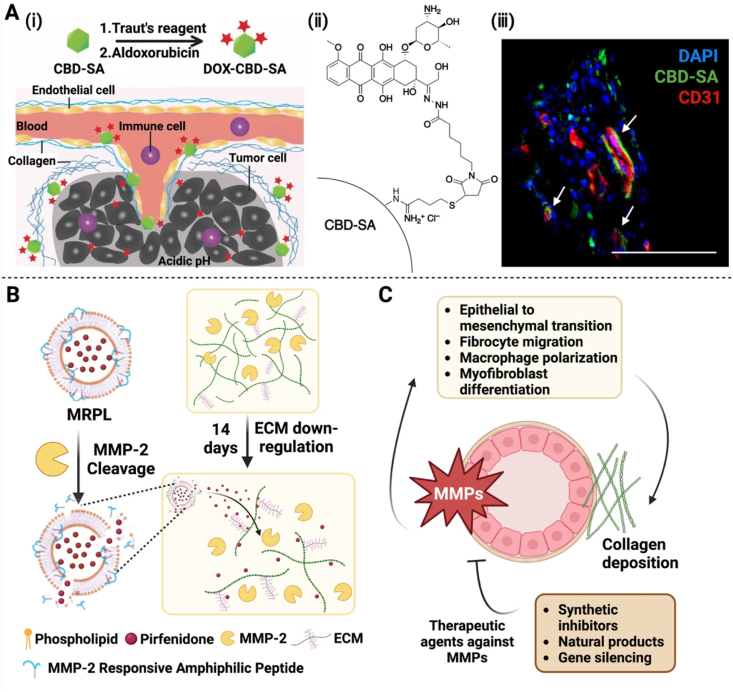
Specific characteristics of the ECM in solid tumors, such as a high level of collagen in the ECM, are often the target for the design of innovative nanodrug delivery systems [101]. Two primary protein types in the tumor ECM are collagen and glycoprotein, and collagen is the most abundant in the animal body. Several multi-functional nanodrug delivery systems with biomacromolecules at their cores have been developed in response to an exceptionally high level of collagen expressed in tumor neovasculature. Biomacromolecules, natural functional components in the body, have been explored in these drug delivery systems, including proteins, polysaccharides, and lipids, due to their high biocompatibility, low immunogenicity, and targeting properties. Albumin, which is commonly used as a nano-drug delivery carrier, has been shown to bind to collagen domains that are rich in the TME (Fig. 7A). Albumin-based nanotherapeutics exhibit anti-tumor effects and they have the ability to penetrate solid tumors [102,103]. Other biological macromolecules that specifically target collagen could be explored as drug delivery nanocarriers. Meanwhile, physical approaches, such as heat generation, could be developed to locally regulating collagen tempo-spatially. High-temperature “melting” by nanoparticles was demonstrated to alter the collagen fibers of tumor tissues and improve the penetration of nanoparticles into tumors [104].
Fig. 7.
Modulation of the tumor microenvironment to achieve deep penetration. (A) Targeting collagen in the tumor microenvironment via DOX-CBD-SA to enable drug accumulation and deep penetration: (i) Schematic representation of the mechanism of action of DOX-CBD-SA. (ii) Structure of the conjugate. iii) Deep penetration achieved by this conjugate. (B) Diagram for the mechanism of peptide hybrid liposomes (MRPL) responsive to MMP-2 in the tumor microenvironment to achieve deep penetration: Illustration of down-regulation of the ECM in pancreatic tumors via MMP-2-responsive MRPL. (C) Schematic for the mechanism of action of MMPs. MMPs play a critical role in remodeling of the extracellular matrix and facilitating deep penetration of therapeutic agents in the tumor microenvironment. Note: CBD, collagen-binding domain; SA, serum albumin; DOX-CBD-SA, Doxorubicin was conjugated to the CBD-SA; MMPs, matrix metalloproteinases; MRPL, a matrix metalloproteinase-2 responsive peptide-hybrid liposome. (Fig. 7A was reprinted with permission from Ref. [102]. Copyright 2019 AAAS. Fig. 7B was adapted with permission from Ref. [62]. Copyright 2017 American Chemical Society. Fig. 7B and C were created with BioRender.com.).
3.1.2. Degrading the ECM
Efforts have been made into targeting multiple sites in the tumor microenvironment by a drug delivery system to achieve deep penetration. In order to effectively penetrate the ECM, weakening the dense structure of the ECM is a promising strategy during the past few years. Moreover, modification of the ECM components or the ECM structure could have an impact on the genesis and growth of tumors. Both physical and biological techniques can be used to degrade the ECM. NIR irradiation as a physical approach was used to promote the release of losartan encapsulated in hollow mesoporous Prussian Blue nanoparticles (HMPBs) to degrade the ECM. It was demonstrated that the penetration depth of DOX in HMPBs was significantly enhanced [105]. In order to improve the overall tumor penetration effect, biological approaches using ECM-specific enzymes have been explored in the biochemical degradation of the ECM. Nanoparticle penetration in tumors can be enhanced by in-situ collagenase degradation of the ECM [62] (Fig. 7B). The collagenase-incorporated nanoscavengers at various sizes were prepared to break through the deep tumor penetration barrier and achieve deep retention in the tumor tissue [106]. Collagenase nanocapsules (Col-nc) derived from engineering polymers with heavy-chain ferritin (HFn) were used to encapsulate chemotherapeutic drug doxorubicin (DOX@HFn). This tactic was found to dramatically increase the penetration of nanoparticles [48]. Hyaluronic acid (HA), one constitutional component of the ECM, is overexpressed in pancreatic cancer [107], which creates a hostile tumor microenvironment for drug delivery. A layered double hydroxide (LDH) nanoplatform modified with hyaluronidase (HAase) and loaded with DOX was developed to constantly release HAase to degrade the ECM in response to the pH gradient in the tumor environment, achieving better tumor penetration of DOX [108].
Matrix metalloproteinases (MMPs) play a critical role in tumor ecology and they are involved in matrix regulation [109]. It has been revealed that they have two essential effects on angiogenesis. Destroying the local ECM by MMPs to make room for endothelial cell growth results in activation of VEGF and fibroblast growth factor (FGF), thus promoting the epithelial-mesenchymal transition. In this context, MMPs can efficiently promote the development of fibrosis [110]. At the same time, MMPs have an inhibitory effect on angiogenesis, because MMPs was first identified as a collagen hydrolase in 1962. MMPs can degrade various proteins in the ECM, and some of the degraded fragments impose an inhibitory effect on angiogenesis, thus MMPs serve a regulatory role in growth signal transduction once the ECM is hydrolyzed by a protease (Fig. 7C). Inhibition of apoptosis and neovascularization by MMPs helps promote tumor invasion and metastasis and creates a favorable milieu for tumor growth. Collaboration between MMPs and tissue inhibitors of metalloproteinases (TIMPs) controls the ECM renewal rate under physiological conditions. In pathological cases, the degradation of the ECM by MMPs facilitates the invasion and metastasis ability of malignant tumors, which is recognized as an essential biochemical cue of tumor cell invasion and metastasis. Among them, MMP-2 has received great attention due to a high expression level of MMP-2 in the tumor ECM. A drug delivery system that is responsive to MMP-2 could be constructed to address the issue of drug impenetrability through the ECM. In this context, PEG-M-PPMT NPs were prepared, which were responsive to three unique features of the tumor microenvironment, including MMP-2. Response to MMP-2 in the ECM resulted in the shrinkage of the NPs [111], eventually achieving effective drug release in solid tumors. The MMP-2 responsive peptide is often employed as the building block for the nanoscale drug delivery system [112]. Multi-functional MMP/folate receptors (FR) micelles loaded with dasatinib (DSB) were developed for tumors with rich MMP-2 and FRs, and it was found that these micelles had excellent targeted uptake and tissue penetration in tumor tissues [[113], [114], [115]]. Another membrane type 1-matrix metalloproteinase (MT1-MPP) lysable peptide was used in a delivery system, DOX-loaded liposomes, the resulted nanomedicine MC T-DOX displayed excellent penetration and tumor cellular uptake performance by indirectly using the angiogenic activity of MMPs [116].
3.1.3. ECM remodeling
Several distinctive molecules and cells play a critical role in matrix regulation, including CAFs, TGF-β, and HIFs. Elucidation of their impact on modulating the matrix environment helps formulation of rational strategies for precise design of nano-drug delivery systems to orchestrate the biological role of these cells and molecules within this matrix.
CAFs are the most abundant and important stromal cells in the TME, and they are the workhorse for generating a solid tumor stroma. Given their critical involvement throughout tumor development, modulation of CAFs represents a viable avenue for intervention of the ECM. TGF- promotes the activation of CAFs, thus TGF-β inhibition could prevent CAFs proliferation and/or differentiation. An illustrative example is FA-EDTA/ICG Lip NPs - a nanoformulation prepared by linking folic acid and polyethyleneimine to the liposome surface via electrostatic interactions, and encapsulating both ethylenediaminetetraacetic acid (EDTA) and indocyanine green (ICG) within the liposome interior. Chelation of EDTA with magnesium ions (Mg2+) was effective in suppressing glycolysis, and the extent of inhibition was determined by the amount of chelated Mg2+. It also down-regulated the production of TGF- by reducing its mRNA expression. A decrease in type I collagen was found by immunofluorescence staining of tumor tissues, thus confirming a decrease in the production of the ECM and achieving microenvironmental regulation. Another two chemical moieties in the nanomedicine contributed to anti-tumor effects: FA helped increase uptake of nanoparticles, and the photosensitizer ICG was induced to produce ROS in response to NIR stimulation. Therefore, the nanoformulation achieved deep tumor penetration as well as displayed a high anti-tumor effect in vivo [117]. In addition, the regulation of CAFs has antitumor implications. DAS@CDC was designed from chondroitin sulfate. The formation of the nanosystem was jointly driven by hydrophobic action and electrostatic interaction. Treatment with DAS@CDC reversed the phenotype of CAFs and reduced the deposition of type I collagen [118], thus achieving enhanced anti-tumor effects in vivo.
An increased IFP, a hallmark in solid tumors, is caused by the compression of blood and lymphatic arteries as a result of hypoxia in the microenvironment, cell proliferation, ECM deposition, and homeostatic imbalance [119]. This increased IFP impedes efficient drug delivery from the circulation to the tumor site. Regulation of CAFs is an effective adjuvant method for drug sensitization and relieve the increase in the IFP. In addition to using a physical method to consume CAFs to facilitate tumor penetration [120], CAFs can be regulated through soluble signal factors. Tranilast, an anti-fibrotic agent, can decrease the expression of VEGF and TGF-, leading to a decrease in active CAFs, and a reduction in the IFP. A nanomedicine, DTX-Ms, included, tranilast and a chemotherapeutic agent doxcetaxel (DTX) since DTX impairs microtubule proteins to achieve tumor-killing effects. This nanomedicine was found to overcome the tumor-promoting barrier of CAFs [121], and normalize microvessels to improve drug penetration and retention, leading to an enhanced drug delivery efficiency.
Hypoxia is one of the hallmarks of tumor tissues and this low oxygen level is ascribed to the growth of aggressive cancer cells [122]. Hypoxia may be a contributing factor for a tumor to develop drug resistance [123,124]. Signal factors induced by hypoxia, HIF-1α and HIF-2α, are discovered to upregulate the expression of collagen hydroxylase P4H and oxidase lysyl oxidase (LOX) in tumor cells [125], thereby promoting collagen degradation and alleviating collagen accumulation, which subsequently facilitates tumor metastasis. Hyperbaric oxygen potentiates (HBOs) have been utilized to reduce the deposition of collagen [126], overcome the hypoxia condition in the tumor and improve the penetration of nanomaterials in the tumor. In the presence of an HBO, the expression of HIF-1α was reduced, which was accompanied with a VEGF expression inhibition rate of 85 % [127]. In this context, the inhibition of HIF-1α is frequently presented as an adjunctive method of anti-tumor therapy [[128], [129], [130]], as HIF-1α inhibitors could indirectly regulate angiogenesis to block the supply of blood nutrients to tumors. However, an effective dose of HIF-1α inhibitors should be applied as an overdose is detrimental to the human body. The advantages and disadvantages of each of the above mentioned strategies are displayed in Table 1.
Table 1.
Advantages and disadvantages of strategies to de-densify ECM.
| Advantages | Disadvantages | Ref. | |
|---|---|---|---|
| Targeting collagen |
|
|
[131] [132] [102] |
| Degrading ECM | Deactivating the pro-tumorigenic function of ECM after degradation via proteases. |
|
[133] [134] [135] [107] [108] |
| ECM remodeling |
|
|
[119] [120] [126] [127] |
3.2. Strategies for changing vascular physiology
3.2.1. Penetration strategies for special biological barriers
The blood-brain barrier (BBB) is a special structure that gradually differentiates during the development phase in the human body and this structure is adapted to a high level of metabolic activity of the brain. Neurons and the surrounding highly branched cerebral vascular network co-evolve [136]. Endothelial cells are the main anatomical core element of the BBB, and the drug permeability through the BBB is significantly reduced because of the gaps between endothelial cells [137]. An interdisciplinary approach is pursued to achieve optimal drug delivery through the BBB for brain cancer.
Glioblastoma multiforme (GBM) is a deadly intracranial tumor. One of promising treatment methods for GBM is the development of a drug delivery system that can penetrate the BBB and reach the intracranial space [138]. Nanoparticles during the BBB transmission process often face challenging issues, such as low affinity, poor selectivity, and immune clearance. Metastatic cancer cell membrane-coated nanoparticles were prepared for treating GBM because the vast majority of tumors have brain metastasis (BMS) complications. The core of the nanoparticles was ICG-loaded polymeric nanoparticles (NPs) prepared from poly(caprolactone) (PCL) and pluronic copolymer F68. The drug delivery system displayed an improved penetration efficiency through the BBB and a promising therapeutic effect for early brain tumors [139].
Ultrasound is an emerging strategy for promoting drug penetration through the BBB. The drug enters the patient bloodstream in the form of microbubbles (MBs). Ultrasound resonance generated from a small ultrasound transmitter on the patient head helps widen the gap between endothelial cells in the BBB, thus facilitating the entry of the drug into the brain site. It was discovered that trastuzumab could be administered to cross the BBB using an image-guided technique. The use of MR-guided focused ultrasound (MRgFUS) could temporarily improve trastuzumab flow through the BBB. It was shown that this procedure could deliver monoclonal antibodies to brain sites without causing any harm [140]. Magnetic resonance image (MRI)-guided focused ultrasound (FUS) and MBs have also been employed to improve the brain penetration effect for nanoscale drug delivery systems. FUS-mediated procedures can dramatically increase tumor interstitial convection and disperse brain penetration nanoparticles (BPNs) [141], so BPNs could overcome the BBB and the blood-brain tumor barrier (BTB) in the brain tumor tissues. BTB usually prevents drugs from entering intracranial tumors, and it is a major obstacle in the treatment of intracranial tumors due to its characteristics such as vascularization and endothelial permeability. As a result, the opening of BTB and BBB in tandem for drug transport is frequently explored [142].
The basement membrane (BM), a critical component of the BBB, maintains the integrity and functionality of the BBB [143]. The BM is often found around vascular endothelial cells and mural cells, and it is a dense, cross-linked, sheet-like extracellular matrix. Despite its lack of cellular compositions, it plays an important role in maintaining physicochemical properties of the vascular environment and the composition of the BBB for its biological integrity [144,145]. The BM [146] not only exhibits as a direct physical barrier for nanoparticle movement, but also affects epithelial cell physiology thus acting as an indirect barrier. A pivotal study revealed the challenge posed by the BM for nanomedicine delivery into tumors [147]. ICG@NPs were used as a photothermal agent for local hyperthermia therapy (LHT). After NPs crossed the endothelial barrier, the perivascular NP pools were found in the subendothelial space without entry into the tumor tissue due to the presence of BM. The number of NPs pools increased after opening of the endothelial linkage gaps under LHT, and leukocytes were recruited by activated platelets into the tumor site, leading to inflammation and neutrophilic infiltration. After immune cells passed through the vascular system [148] and neutrophils migrated through the BM, NPs pools erupted into the tumor site. The synergistic effect of LHT and the dynamic opening of the endothelial gaps upon migration of neutrophils through the BM ultimately results in effective drug delivery. The development of nanomedicine for anti-tumor treatment should consider the complex barrier properties of the BM. By demystifying and exploiting the characteristics of the BM, promising strategies could be formulated to improve the intratumoral delivery efficiency of nanomedicine.
3.2.2. Vascular physiological changes based on RNS and NO
Nitric oxide (NO) is considered as a communication medium between cells and it plays an important role in the human body. All cells in the human body can produce NO. Angiogenesis is a physiological process to develop new blood vessel networks required for normal body function. NO is a crucial signaling molecule in the control of local angiotasis and it is rapidly produced by endothelial cells via a unique isotype of nitric oxide synthase (eNOS) [149]. The vasodilator property of NO was initially found in 1980. This property has been explored to help drug penetration. It has been reported that solid tumors obtain their nutrients from capillaries to promote abnormal proliferation and metastasis of tumor cells. Dilation of deep tumor blood vessels in the tumor parenchyma with aberrant blood vessels via regulating NO in order to improve the penetration of drugs into solid tumors could be a promising approach.
DNP nanoparticles were designed to incorporate docosahexaenoic acid (DHA) and nicorandil (NI). In reaction to glutathione (GSH) in tumor cells, the released DHA and NI from the DNP nanoparticles produced reactive oxygen species (ROS) and NO. NO-mediated vasodilation assisted in hypoxia relief and stress elevation in tumors, thereby boosting ROS-dependent toxicity and enhancing deep tumor penetration. Meanwhile, lethal reactive nitrogen species (RNS) was generated via ROS and NO in situ [150,151], and RNS could cause DNA strand damage and inhibit DNA repair enzymes, thus leading to cell apoptosis [152]. This approach allowed gradual penetration of drugs into the tumor, achieving the anti-tumor effect through cascade amplification. Similarly, intelligent nanoparticles called IDDHN consists of two major components, IDD and HN. IDD is a derivative prodrug of DOX and ICG, and HN refers to HAase-sensitized and NO donor-modified HA shells. IDDHN were irradiated to promote the release of NO in-situ by a laser, thereby extending the depth at which drugs were absorbed [153]. Apart from the vasodilation effect of NO on blood vessels, low-dose radiotherapy to increase the level of NO could enhance transient vascular permeability and ultimately increase drug penetration [154].
3.3. Structural changes
Structural design of nanomedicine plays a critical role in enhancing drug penetration into tumors. Incorporating various structural moieties to manipulate the size, shape, charge, surface functionalities, and stimuli-responsiveness [155] into a nanoparticle assembly allows for improved drug stability during transport, selective drug delivery, and effective tumor penetration, ultimately enhancing therapeutic efficacy. By carefully considering and optimizing these structural moieties, nanodrug delivery systems can be designed to efficiently target tumor sites, overcome biological barriers, and enhance drug penetration within tumors. For example, the hydrophile-lipophile balance (HLB) plays an important role in the self-assembly behavior of nanoparticles and amphiphilic molecules [156,157]. Manipulating the HLB of amphiphilic nanoparticles not only enables them to spontaneously self-assemble into ordered structures such as micelles, vesicles, or lipid bilayers [158], ensuring stability during transport before reaching tumor regions and facilitating cellular uptake and internalization. In addition, size modulation of nanodrug delivery systems is the most sought strategy for their preferential accumulation in the tumor microenvironment through the well-known “enhanced permeability and retention” (EPR) effect, which is characterized by the leaky vasculature and impaired lymphatic drainage in solid tumors [159,160]. However, these structural design strategies are primarily based on passive tumor penetration driven by the EPR effect, and the degree of tumor penetration of nanodrug delivery systems remains very disappointed. The strategies for different kinds of structural changes are displayed in Table 2.
Table 2.
Structural change strategies.
| System name | Mechanisms | Experimental animal model | Size changes (nm) | Penetration Model/imaging methods in Tumors | Ref. | |
|---|---|---|---|---|---|---|
| External physical stimulation |
HBPTK-Ce6@CPT | ROS & light triggered size-reduction | HT29 xenografts tumor-bearing nude mice | 210-110-40 | HT29 MCSs and frozen sections | [182] |
| PDA@GNRs-DOX/Ce6 | NIR light triggered size conversion | U14 tumor-bearing mice | 440-150-50 | Real-time thermal imaging in vivo | [181] | |
| ICG@HSA-Azo-HP | Photothermal triggered size-shrinkage | 4T1 tumor-bearing mice | 140–11 | 4T1 multicellular tumor spheroids (MTSs) and frozen sections | [184] | |
| DAT-PPED&F | Magnetic actuated TAT reactivation | MDA-MB-231 xenograft tumor tissue | 180-100 (acceptable deformation penetration diameter) | Ex vivo visualized via CLSM | [54] | |
| ADM-GNMF |
Ultrasound triggered response |
/ |
46 12-1212 35 (drug release) |
/ |
[190] |
|
| Tumor internal environment response | PDPP@D | Neighboring effect triggered by pH/redox conditions | 4T1tumor-bearing mice | 60 | 4T1 MTS and frozen sections | [42] |
| GFLG-DP | CTSB triggered stealthy-to-sticky conversion | 4T1 tumor-bearing mice | 150-80-20 | 4T1 MCSs and in vivo real-time imaging | [205] | |
| IDDHN | HAase triggered size shrinkage | 4T1 breast cancer-bearing mice | 264-(most at 29–50) | 4T1 MCSs and ex vivo imaging | [153] | |
| UAMSN@Gel-PEG | MMP-2 triggered size shrinkage | 4T1 tumor-bearing mice | 76–6.5 | Ex vivo and visualized by CLSM | [63] | |
| iCluster/Pt | Intrinsic tumor extracellular acidity triggered structural alteration | BxPC-3 pancreatic tumor-bearing mude mice | 100-(∼5) (release small PAMAM prodrugs) | BxPC-3 MCSs and histological analyses | [204] | |
| SCNs/Pt | pH triggered size transition | BxPC-3 xenografts tumor-bearing mice | 80-(less than 10) | BxPC-3 MCSs and real-time imaging by intravital CLSM | [162] | |
| SA-DOX@HA-CD | pH triggered self-disintegration of nanoassemblies | 4T1 tumor-bearing BALB/c mice | (150–200)-(30–40) | 4T1 tumor spheroids and fluorescence imaging | [203] |
Characteristic factors in the tumor microenvironment, such as ECM deposition and an IFP, have been revealed to resist the penetration of nanosystems into deep regions of tumors away from the vasculature [161,162]. Therefore, active strategies have been proposed to enhance tumor penetration through rational design of nanomedicine by exploitation of tumor-specific physiological and pathological features. For example, a size-switching strategy has been proposed: larger nanoparticles transform into smaller ones upon entering the tumor [[163], [164], [165], [166]], effectively increasing the depth of drug penetration [167,168]. Additionally the design of nanomedicine with charge-reversal properties has been reported [[169], [170], [171], [172]]. Maintaining neutral or weakly negative surface charges in the circulation and switching to positive charges within tumors can improve tumor penetration while reducing toxicity and uptake by the reticuloendothelial system. Furthermore, self-assembled nanomedicines can maintain their assembly structures in physiological conditions for drug delivery and disassemble within tumors, promoting drug penetration [[173], [174], [175], [176]]. These structure-changeable strategies can be externally triggered using photothermal ultrasound or magnetic fields, or more specifically driven by tumor microenvironments, offering significant potential for enhancing tumor penetration.
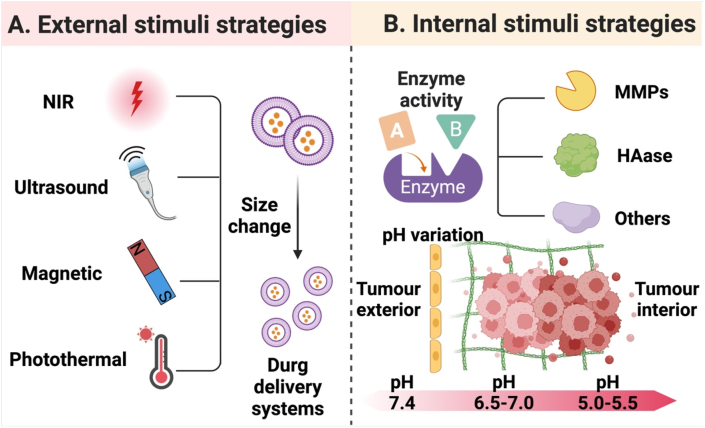
This section discusses active tumor penetration strategies in nanomedicine by exploiting external stimuli or features of the tumor microenvironment to offer structural variations of nanodrug delivery systems. The strategies covered in this section are briefly illustrated in Fig. 8.
Fig. 8.
Illustration of multiple strategies for improving tumor penetration of nanomedicine. (A) Utilization of external stimuli, such as NIR light, ultrasound, magnetic fields and other physical sources to induce in vivo response of nanomedicine, leading to its size shrinkage to improve its antitumor therapeutic effect. (B) Utilization of internal stimuli to strengthen the antitumor effect of nanomedicine by improving drug penetration through the action of enzymes like MMPs and HAase or at a low pH in the tumor microenvironment. (Fig. 8A and B were created with BioRender.com.).
3.3.1. External stimuli
Near-infrared (NIR) light is an example of an external stimulus [[177], [178], [179], [180]]. “Remote-controlled cluster bombs” [181] with the ability of deep penetration into solid tumors were designed for tumor therapeutic treatment. The cluster bombs, HBPTK-Ce6 NPs, were induced to generate a high level of ROS at the tumor site under 660 nm laser irradiation. ROS-responsiveness of these NPs led to programmed size reduction for deep tumor penetration [182]. Although it was not the primary method utilized in this investigation to control size changes of NPs, light-stimulus response was a quick and efficient strategy to promote nanoparticle degradation [181]. Both SNPICG/Ce6 [183] and a photothermal-responsive albumin nanocluster (ICG@HSA-Azo-HP) [184] were developed based on an anoxic microenvironment and their tumor penetration was gradually modulated. Triggers for the structural change of these nanoparticles were either directly or indirectly from light.
External stimuli also include ultrasound [185], magnetism [[186], [187], [188]], and other sources. Deformable nanocomplexes, DAT-PPED&F, could achieve deep tumor penetration through magnetic control [189] and reprogramming. Ultrasonography was applied to induce release of an anticancer drug, adriamycin (ADM) from adriamycin gelatin nanogel modified with fluoride anion (ADM-GNMF) [190]. Moreover, ultrasound guidance can dramatically enhance the drug delivery efficiency [191,192]. Ultrasound-induced cationic nanodroplets with variable sizes have been shown to enhance tissue and cell permeability [193]. All of the stimuli mentioned above cause the nanoparticles to deform or undergo structural changes, resulting in the release of the encapsulated drug, thus achieving effective treatment.
3.3.2. Internal stimuli
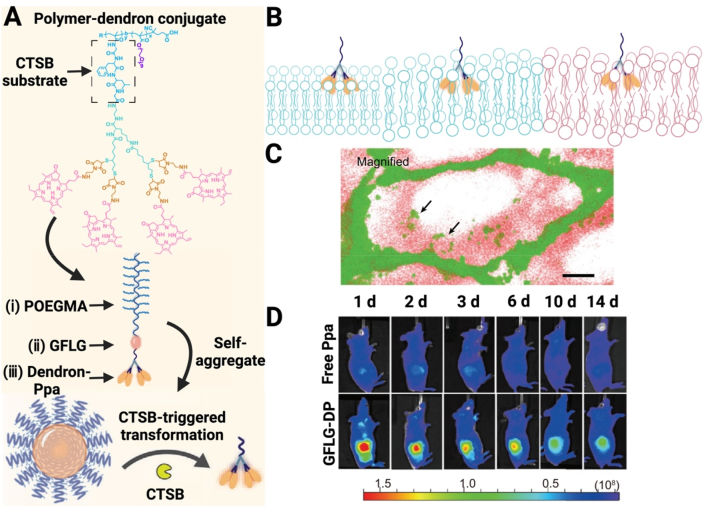
Various specific enzymes and pH gradients in the tumor tissue are examples of internal stimuli [[194], [195], [196], [197]]. Due to a moderate acidity of the tumor microenvironment, pH variations are often used as a stimulus to induce changes in the size of nanoparticles to promote drug penetration [[198], [199], [200]]. pH-sensitive chemical bonds are often employed in polymers [201,202], or inorganic compounds for therapy and diagnosis, and breakage of these chemical bonds in response to an acidic environment in the tumor could lead to size changes for successful deep penetration of the tumor. An instant acid-responsive size-changeable drug delivery system (SA-DOX@HA-CD) was developed. This nanosystem could rapidly break down into monomers in a mimicking microenvironment (pH6.5), and this response to this immediate pH resulted in a 100 % disassembly efficiency for the nanosystem [203]. Ultra-pH-sensitive size-switchable SCNs/Pt was prepared for improved tumor penetration and cancer treatment [162]. A multifunctional two-component NP (PP NP) driven by electrostatic interactions, PEG-b-PAMAM/Pt, was constructed. This NP could disassemble in response to a slightly acidic environment of tumors but re-assembled into small NPs under infrared laser irradiation [64]. Endogenous activation by the tumor microenvironment promoted structural alterations of the nanoparticle. After structure alterations, the PAMAM dendrimer prodrugs remained at 5 nm in size, and they exhibited excellent penetration capacity [204] (Fig. 9). Enzymes as another stimulus are widely employed to trigger the size change of nanoparticles. For example, given abundant MMP-2 in the tumor microenvironment, MMP-2 responsive nanoparticles with variable sizes, UAMSN@Gel-PEG, were prepared. Gelatin was used as the substrate of MMP-2 in the nanoparticles. When the nanoparticles accumulated at the tumor site due to the EPR effect, MMP-2 rapidly digested gelatin in the nanoparticles, facilitating deep penetration of UAMSn-loaded ammonia borane into the tumor [63]. Size shrinkage of intelligent nanoparticles, IDDHN [153], was triggered by HAase in the tumor microenvironment.
Fig. 9.
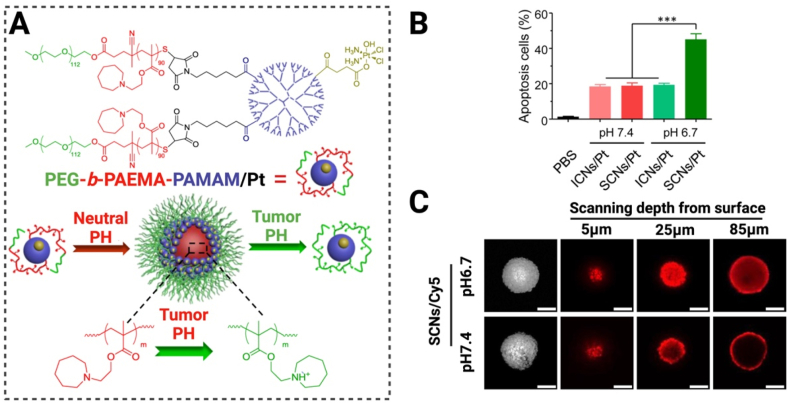
(A) Schematic representation of the mode of action of positively charged nanoparticles (PEG-b-PAMAM/Pt) under acidic conditions, scale bar: 100 m. (B) Induction of apoptosis by nanoparticles under different pH environments. (C) Differential permeability of Cy5-labeled nanoparticles under different pH conditions. (Fig. 9A–C were reprinted with permission from Ref. [162]. Copyright 2016 American Chemical Society.).
3.4. Active tumor penetration
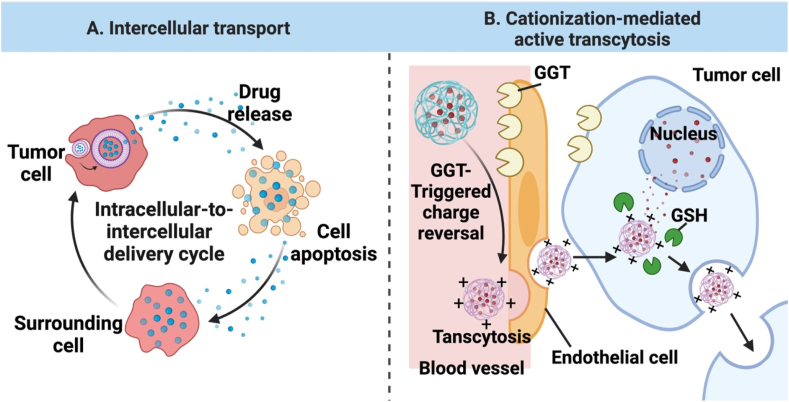
The concept of “active tumor penetration” is emerging as a promising approach to addressing the challenge for in vivo delivery of nanomedicine. This approach enhances tumor penetration by modulating structural properties of nanomedicine such as morphology, size, and charge or incorporating specific ligands/moieties on the surface. Major strategies have been proposed for this approach, including: 1) modification with cell-penetrating peptides; 2) intercellular transport; 3) cationization-mediated active transcytosis; and 4) organelle-affinitive transfer (OAT).
3.4.1. Cell-penetrating peptides
Specific ligands have been employed to successfully achieve active tumor targeting, and these ligands can be explored to enhance tumor penetration of nanomedicine through surface modification. This active tumor penetration strategy is evolved from the active targeting ligand modification method. A common method of implementing this strategy is the use of cell-penetrating peptides (CPPs).
CPPs are short peptide sequences, typically consisting of 5–30 amino acids. They possess remarkable membrane crossing capabilities, allowing CPPs-modified nanomedicine to traverse biological membranes and enter cells [[206], [207], [208], [209]]. After attachment of CPPs to the surface of nanocarriers, such as liposomes, through covalent or non-covalent surface modification techniques, cell-penetrating properties of CPPs could facilitate efficient interaction between CPPs-modified nanocarriers and tumor cell membranes [210,211]. CPPs can also be strategically camouflaged to avoid non-specific internalization [212]. Therefore, the incorporation of CPPs to nanocarriers can help deliver drug molecules in the nanocarriers into deeply-seated tumors with an improved efficiency in overcoming the tumor cell membrane barrier and an elevated level of transportation and penetration within the stromal tumor [213]. The transmembrane mechanisms of CPPs include direct penetration, entotic absorption, and an inverted colloidal model. For example, smart nanoparticles based on CPPs were prepared in a neutral environment using a ligand-switchable method. Upon exposure to an acidic environment, CPPs were concealed in the blood under a PEG corona and they were exposed to the tumor cell membrane upon arrival of tumor sites after the PEG corona was deshielded from the nanocarrier [214]. PTX-encapsulated, CPP-modified transfersomes (CTs) (PTX-CTs) were developed for the treatment of cutaneous melanoma.
Arginine-rich CPPs, which readily accumulate in selected organs, are the most commonly utilized CPPs for drug delivery nanocarriers [215]. In contrast, R8, another representative CPP, is often used for penetration of tumor xenografts. R8 is a stearylated product at the N-terminus of CPPs with stearyl-octaarginine, and it is useful for the delivery of nucleic acid pharmaceuticals (NAPs) [216]. Due to its highly internalized capacity and versatility in structural modification, R8 has been widely adopted for anti-tumor drug nanocarriers [215]. Derived from a R7 (RRRRRWW)-functionalized CPP, a P9 (RRRRRWWPP) was recently reported to complex with PTX, and this complex system, P9-PTx, displayed low toxicity and great biocompatibility [217]. The affinity between PTX and a CPP could also be achieved via double-proline in the CPP. However, direct application of CPPs is hampered by their non-specific targeting effect and their potential to be recognized and cleared by the RES. Improving the targeting ability of CPPs has been explored. A CPP that recognized the RRRRRRRRRRRRDGR (R8-dGR) dual receptor was developed to specifically target the integrin αvβ3 and NRP-1 receptors, thus facilitating penetration in solid tumors [218]. A multifunctional liposome (CL-R8-LP) was developed by incorporating R8. R8 was able to penetrate the cytoplasmic membrane barrier during liposomal drug delivery. Cholesterol-anchored reduction-sensitive PEG was conjugated to mitigate the clearance of R8-functionalized liposomes. This surface modification method increased non-specific CPP osmosis and improved tumor targeting [219].
In recent years, the iRGD peptide has been widely utilized as a targeted peptide for tumor imaging. It is a family member of the tumor penetrating peptide (TPP) and it has a unique amino acid sequence. Due to its great affinity and precise binding, it can help deliver both imaging agents and anticancer drugs deep inside tumors. Dual-channel fluorescent iRGD was developed to demonstrate that the conjugation of iRGD to pharmaceuticals could improve the penetration of nano-drugs in tumors [220]. Recent studies have revealed that iRGD penetrates tumors through the β5 integrin-mediated molecular pathway. The iRGD-conjugated nanomedicine could successfully reach deep tumor tissues through the fiber network of pancreatic tumors [221]. This polypeptide peptide was found to bind to neuropilin [222], one of the highly expressed proteins in pancreatic cancer cells. The binding process promoted endocytosis of the nanomedicine and vesicle trafficking inside cells. An iRGD-based pattern peptide amphiphile self-assembled into spherical nanovesicles. The nanoplatform could improve photodynamic therapy as well as intratumoral accumulation [223]. By evaluating a 3D cell ball penetration model, iRGD and pH-sensitive fluorocarbons were incorporated into a nanocarrier with enhanced intratumoral accumulation in the original breast cancer tumor [224]. It was also discovered in a multicellular sphere model that iRGD increased the number of tumor-specific T cells when it was conjugated to T cell surfaces [225].
Although CPPs are often considered to help penetrate solid tumors due to their effective membrane penetration, the general applicability of CPP-mediated penetration is constrained by its non-selectivity and lack of receptor specificity. CPPs could be loaded onto other nanoscale carriers with specific selectivity towards tumor cells. Alternatively, the primary component of the CPP or its structural design could be manipulated to enhance its selectivity and specificity to tumor cells. The TPP, represented by iRGD, could be versatilely incorporated to nanoscale carriers to enhance intratumor accumulation and intertumor penetration.
3.4.2. Intercellular transport
Intercellular communication is critical for maintaining physiological function and influencing pathological development. The primary method by which neighboring cells communicate is through the intercellular transport of substances, including inorganic ions, neurotransmitters, and small protein molecules. Recently, novel methods of communication have been identified, such as tunneling nanotubes [226,227] (TNTs) and extracellular vesicles (EVs). The identification of EVs reveals that cells can transfer materials between each other via vesicular transport [228,229], providing a biological basis for designing nanomedicines that exploit intercellular transport for enhanced tumor penetration.
In the context of drug delivery systems in tumors, this phenomenon is also known as the neighboring effect (Fig. 11A). This occurs when tumor cells act as an in-situ drug reservoir after they engulf drug-loaded nanomedicine [230]. Drug-loaded nanomedicine uptaken by tumor cells close to the vascular system often promotes their apoptosis, and the engulfed drug is released from dying cells. Recent studies indicates that apoptotic bodies (ApoBDs), specific extracellular vesicles involved in intercellular communication, mediate this transport process, thereby facilitating drug transport between cells. When tumor cells undergo apoptosis, the ApoBDs secreted during tumor cell apoptosis can store the engulfed drugs by tumor cells. These ApoBDs can then efficiently deliver the entrapped drug into surrounding cells through extensive phagocytosis, achieving active intercellular drug transport [231]. This strategy can enhance drug penetration and distribution beyond the immediate vicinity of the vascular system in the tumor, thereby improving the therapeutic efficacy. It is especially appealing in tumors with heterogeneous cell distribution [232,233]. However, the role of the ApoBDs-mediated intercellular transport (also known as the ApoBDs-mediated enhanced neighboring effect) in promoting tumor penetration is often impaired, which may be due to drug consumption and/or drug resistance in hypoxic tumor cells. Novel approaches have been explored to effectively transport a high dose of nanomedicine between cells after tumor cell death.
Fig. 11.
Illustration of (A) Intercellular transfer (also known as neighboring effect) and (B) Cationization-mediated active transcytosis. Note: GGT, a membrane γ-glutamyl transpeptidase; GSH, γ-glutamylamides of glutathione. (Figs. 11A and B were created with BioRender.com.).
To achieve the best penetration-enhancing effect, nanomedicine could be designed to release its therapeutic agent(s) in a controlled and sustained manner. This ensures that the therapeutic payload is available for diffusion over an extended period, repeatedly infecting more neighboring tumor cells. In addition to traditional chemotherapy to kill tumor cells, radiotherapy, photodynamic therapy [234], and other apoptosis-inducing therapies can be employed [235]. Chemotherapeutic nanomedicine could enhance drug penetration in solid tumors and amplify their therapeutic effects through the neighboring effect (Fig. 10). Combining different therapeutic agents within a nanomedicine can potentiate this process. For example, a recent study has reported the use of self-assembled PEGylated dendritic peptide-pyropheophorbide a (Ppa) conjugates (PDPP) to encapsulate the chemotherapeutic drug DOX, resulting in a pH- and redox-responsive nanomedicine, PDPP@D [42]. It has been demonstrated that the nanomedicine significantly promoted drug release from dead tumor cells and the released drug retained its active form to exert cytotoxicity on neighboring tumor cells. This notable intercellular drug transport was attributed to the drug release mechanism of the nanomedicine: a low intracellular/lysosomal pH rapidly protonated DOX, and a high level of cytoplasmic GSH triggered disulfide bond cleavage, leading to the sustained release of DOX and Ppa from PDPP@D. Moreover, the combination of photodynamic therapy and chemotherapy more effectively induced irreversible ER stress in tumor cells and promoted apoptosis, resulting in enhanced drug penetration into tumor tissues and a significantly prolonged drug residence time.
Fig. 10.
(A) Fluorescence confocal microscopy images capturing gradual infiltration of PDPP@D nanoparticles from external blood vessels of the tumor to the inside of the tumor. (B) Quantitative fluorescence intensity of Ppa for free Ppa, PDPP and PDPP@D in tumors of mice at different time points (n = 3). (Figs. 10A and B were reprinted with permission from Ref. [42]. Copyright 2022 Wiley.).
Consequently, by rationally optimizing nanodrug strategies, such as combination therapy, it's possible to amplify the intercellular drug transport, enhancing drug penetration and eradicating tumors. Therefore, inducing and enhancing the intercellular drug transport can be viewed as a strategy to promote active tumor penetration. This approach holds potential for innovative therapeutic methods, aiding in the battle against cancer by maximizing the reach and efficacy of treatments.
3.4.3. Cationization-mediated active transcytosis
Transcytosis, a critical subtype of vesicle-mediated transport, has been explored as a mechanism for drug delivery. It can facilitate active penetration of nanomedicine into solid tumors through a series of biological barriers [236], including the tumor microenvironment, ultimately reaching deeper tumor sites [237]. Absorptive-mediated transcytosis (AMT), a specific type of transcytosis, is executed by recruiting nanomedicine onto a cell surface, triggering cellular internalization and transcellular transport [238]. This process is particularly attracting because nanocarriers in nanomedicine can penetrate multiple cell layers in tumor tissues [239,240]. To ensure safety and efficacy of nanomedicine and promote tumor penetration through AMT, a sufficiently high cellular uptake efficiency is critical for AMT. Specific surface properties of nanocarriers in nanomedicine can be manipulated to help efficiently populate on the surface of tumor cells, thus promoting endocytosis of nanomedicine into the cells. For example, the positive charge of cationization can mediate highly efficient AMT by facilitating electrostatic interactions with tumor cell membranes.
Cationic nanomedicine has advantages in triggering transcytosis-mediated active tumor penetration [241] (Fig. 11B). A positive surface charge of nanomedicine facilitates its interaction with negatively charged cell membranes of tumor cells, promoting their adsorption and internalization [242]. After adsorption onto the cell surface, cationic nanocarriers can be internalized via endocytosis and then transported across the cell through transcytosis. Furthermore, the proton sponge effect from the cationic nanocarriers helps them escape from late endosomes and lysosomes into the cytoplasm [243], avoiding lysosomal degradation. However, a cationic surface charge of nanocarriers is unfavorable during blood transport, since these carriers may be adsorbed to negatively-charged macrophages and the formed complex could be cleared before reaching the tumor site. Therefore, the charge reversal strategy is commonly used to address this issue. For instance, a membrane γ-glutamyl transpeptidase (GGT)-responsive polymer-drug conjugate [244], PBEAGACy5-CPT, was developed. This nanocarrier was electrically neutral in the blood after it was masked with GGT-responsive groups. A positive charge surface was exposed after the GGT-responsive groups were removed by GGT in the tumor environment, inducing cationization-initiated transcytosis. In this study, GGT-responsive charge reversal facilitated transcytosis of PBEAGACy5-CPT through tumor vascular endothelial cells, thus actively transporting the nanomedicine deeply into the tumor tissue. Once inside the tumor, the AMT process continued to facilitate deeper penetration into the solid tumor [245]. Overall, nanomedicine can be effectively designed to achieve high cellular uptake efficiency and facilitate active transcytosis through charge reversal strategy and cationization-mediated mechanisms, thereby enabling improved tumor penetration and therapeutic efficacy.
3.4.4. Organelle-affinitive transfer
An emerging approach for targeted cancer therapy is to target organelles within tumor cells by nanomedicine. The overall goal of this approach is to revolutionize cancer treatment by maximizing the therapeutic effect while minimizing damage to healthy tissues. Nanomedicine is elegantly designed to effectively target organelles within cancer cells through various strategies including structural manipulation to control release patterns. These strategies take advantage of unique properties of tumor cells and their organelles, such as mitochondria [246], the endoplasmic reticulum (ER) [247], endosomes [248], lysosomes [[249], [250], [251]], and nuclei [252].
It is noted that organelles within cells are not isolated entities within the cytosol [253]. Extensive exchange of substances and information occurs between cell membranes and organelles, as well as between different organelles [254]. These exchange processes are facilitated not only by vesicular transport but also through contact areas between these membrane structures known as ER and inter-organelle membrane contact sites (MCSs) [255]. It is evident that the ER plays a pivotal role at contact sites between the plasma membrane (PM)-the organelle membrane or between inter-organelle membranes [256]. Contact points are formed with the cell membrane, vesicles like endosomes [257], and other organelles [258]. These contact points are essential for various cellular processes [[259], [260], [261]], such as lipid transfer [262], calcium signaling [263], and communication between the plasma membrane and other organelles like mitochondria. The communication helps maintain cellular balance and ensures efficient cellular operations. These membrane contacts between organelles are dynamic, adaptable for various cellular functions and processes [[264], [265], [266]]. Such processes include cellular endocytosis of nanoparticles and the transport of vesicles and lipids within the cell [[267], [268], [269]]. This suggests that the ER could play a crucial role in active tumor penetration for nanomedicine.
We have recently introduced a tumor penetration strategy by exploiting the affinity between the ER/PM membrane for active transfer [205,270], tentatively termed as “organelle-affinitive transfer” (OAT). It is a unique type of vesicular transport that differs dramatically from a typical transcytosis process. First, the cellular internalization process mediated by this method is in a form of non-clathrin endocytosis, while a transcytosis process often relies on classical clathrin-coated pits. Second, during the organelle-affinitive transfer process, once nanoparticles are internalized into the cell, they bypass the endosome/lysosome pathway and enter the ER directly. Therefore, the nanocarriers may not require a significantly positive charge for lysosomal escape. These properties could potentially balance the in vivo drug stability, barrier circumvention, tumor penetration, and mitigation of side effects. Such design of nanocarriers based on the OAT process could be a new avenue to promote tumor penetration of nanomedicine.
To realize the OAT process, we synthesized a unique amphiphilic polymer-dendron conjugate, POEGMA-b-p(GFLG-dendron-Ppa) (GFLG-DP) [205,270] (Fig. 12A and B). GFLG-DP was enzymatically responsive to overexpressed cathepsin B in tumor cells. After enzymatic cleavage of GFLG-DP, dendron-Ppa was obtained as a degradation product of GFLG-DP. The unique molecule, dendron-Ppa, exhibited high affinity for both cell membranes and the ER membrane. The dendron-Ppa segment of this nanocarrier showed pronounced affinity for lipid rafts on tumor cell membranes (Fig. 12C). Since lipid rafts are a special microdomain on the cell membrane enriched in cholesterol, sphingolipids, and specific proteins, preferential accumulation of GFLG-DP in lipid rafts significantly enhanced their interaction with the cell membrane, thereby improving their selective internalization efficiency into tumor cells. Moreover, dendron-Ppa with a dendritic structure as the predominant hydrophobic component displayed amphiphilicity, which facilitated embedding of the dendron-Ppa segment into the cell membrane, leading to the formation of fused vesicle structures during cellular endocytosis. The fused vesicles contained both dendron-Ppa and the membrane structure of the internalized tumor cell, while the membrane structure bore a resemblance to EVs. However, the vesicles mediated by dendron-Ppa were not derived from extracted or engineered EVs, actually they formed in situ in tumors during the internalization process. We observed that after internalization, dendron-Ppa rapidly entered the ER without co-localizing with lysosomes or other organelles. This suggested that the vesicles formed by dendron-Ppa had high affinity for the ER membrane. Given the important role of the ER in cellular endocytosis and secretion, dendron-Ppa could hold exceptional tumor penetration capabilities (Fig. 12D). Continuous intracellular and extracellular transport of dendron-Ppa helped its penetration into deeper layers of tumor tissues distal to blood vessels. In addition, dendron-Ppa also functioned as a photosensitizer to induce strong perturbation on the plasm and ER membranes through PDT, exerting ER stress and disrupting intracellular hemostasis. The perturbation may also promote deeper tumor tissue penetration of dendron-Ppa through intercellular interactions. The proposed OAT process has been demonstrated to achieve targeted drug delivery and remarkable tumor penetration through a special transfer mechanism.
Fig. 12.
(A) Illustration of the design process for CTSB-triggered GFLG-DP: stealth-to-sticky structural transformation of GFLG-DP because of enzymatic hydrolysis of the GFLG substrate by abundantly expressed CTSB in tumor tissues. (B) Interaction between tumor cell membranes and dendron-Ppa released from GFLG-DP: After internalization, dendron-Ppa released from GFLG-DP in response to CTSB is distributed on the ER, inducing the ER stress to disrupt ER homeostasis. Upon laser irradiation, strong oxidative stresses due to a high level of ROS induce apoptosis. (C) STED confocal microscopy image for 4T1 Cells after incubation with GFLG-DP, scale bars: 2 μm. (D) Accumulation of free Ppa and GFLG-DP NPs at tumor sites via an in vivo imaging system. Note: CTSB, cathepsin B; ER, endoplasmic reticulum; STED, stimulated emission depletion. (Figs. 12A–D were reprinted with permission from Ref. [205]. Copyright 2022 Wiley.).
4. Application of other artificially modified nanocarriers
Many challenging issues arise in the application of nanoscale drug delivery systems in the human body. They need to surmount a series of biological barriers, such as the reticuloendothelial phagocytosis system (RES), and an IFP within the ECM. A recent bio-inspired “stealth” strategy is functionalization of nanoparticles by coating them with a cell membrane [271]. Cell membrane-encapsulated nanoparticles have become a novel class of nanocarriers with a core-shell structure [272]. Common membrane-coating techniques involve mechanical or ultrasonic treatment to cover a nanocore with a membrane, resulting in a spontaneous “core-shell” structure. Cell membrane-coated nanoparticles (CM-NPs) not only exhibit excellent biocompatibility, but also offer superior protection of therapeutic drugs in the core and promote tumor penetration.
By modifying their surfaces, engineered nanoparticles can extend their blood half-life by mitigating macrophage phagocytosis and its mediated clearance. The most common approaches include PEGylated functionalization and surface modifications [58,[273], [274], [275]]. Among surface modification methods, surface functional groups derived from natural materials show their advantages over synthetically derived moieties. Extracellular vesicles (EVs), membrane-like vesicles released by cells, are considered to have great potential as a coat for surface modification to facilitate tumor penetration. In comparison to synthetic nanocarriers, EVs have better targetability, safety, and drug metabolism. A few special surface engineering techniques for promoting tumor penetration will be covered in this section, including synthetic liposomes with natural membrane-mimicking properties, and naturally-derived exosomes released from cells. Functionalized liposome and exosome are characterized by both natural and synthetic components, which are next disassembled and analyzed from the perspective of CM-NPs.
4.1. Liposomes
Liposomes have been extensively used as a synthetic nanocarrier because their surfaces have similar performance as natural cell membranes. They are closed vesicles with a bilayer structure. The diameter of liposomes is between 30 and 150 nm. They are often composed of phospholipids, cholesterol, PEG, and other substances; they have low immunogenicity and low toxicity; and they can cross biological barriers. Lipid nanoparticles (LNP) in the COVID-19 mRNA vaccines are the most recently clinically applied liposomes [[276], [277], [278], [279]]. Although liposomes exhibit the EPR effect and they can extend the circulation of drugs in the liposomes, this effect is not adequate for deep drug penetration [280]. Multi-functional liposome nanoparticles are often created to achieve effective drug permeability.
A liposome typically acts as a skeleton and functional molecules including enzymes or PEG can be attached to its surface [62,281]. Incorporation of functional moieties to a liposome could allow the liposome to become responsive to a range of internal or external stimuli, or become more accessible to deep tissues, thereby improving drug penetration in solid tumors. PEG is usually supplied on the surface of a liposome to build a functional stealth nanocarrier [282,283] and other surface engineering techniques are combined with PEGylation to promote tumor penetration (Fig. 13A). In the combination therapy for triple-negative breast cancer, a multi-stimuli sensitive liposome (ELTSL) was a complex liposome vector modified by PEG [281]. A size and charge dual-conversional gemcitabine prodrug-integrated liposomal nanodroplet (SCGLN) was created. Orthotopic BxPC3 pancreatic ductal adenocarcinoma was selected in this study, and the SCGLN loaded with gemcitabine showed great tumor permeability [29]. Cell penetration enhancers (CPEs) were added to PEGylated doxorubicin liposomes (PLD) [284] to make them more effective in penetrating tumors. A MMP-2 responsive peptide-hybrid liposome (MRPL) was constructed in response to overexpressed MMP-2 in the tumor microenvironment [62].
Fig. 13.
Examples of surface-engineered nanoparticles and their associated mechanisms. (A) Illustration of a general structure of liposomes for drug delivery. (B) Three mechanisms of action of exosomes: (i) Endocytosis. Exosomes are internalized by recipient cells to release cargo molecules. (ii) Membrane fusion. Exosome membranes fuse with recipient cell membranes, resulting in the transfer of cargo molecules, and (iii) Receptor-mediated specific binding. Exosomes selectively bind to recipient cells through receptor-ligand interactions, enabling targeted delivery of cargo molecules. (Figs. 13A and B were created with BioRender.com.).
By enhancing the EPR effect, nitric oxide donors on liposomes facilitated the accumulation of liposomes in the tumor tissue [285]. A stimuli-responsive polysaccharide enveloped liposome carrier (HCLR) was constructed [286], where chitosan (CS) and HA were added layer-by-layer to liposomes. The surface charge of liposomes could play a significant role on tumor penetration. Positively charged liposomes, for instance, displayed greater binding ability [287]. By modulating the surface charge of liposomes with different components [288], cationic PEGylated nanoparticles exhibited effective tumor inhibition. Apart from incorporating functional moieties to a liposome, structural or compositional manipulation of liposomes could facilitate their penetration into solid tumors. For instance, variations in the rigidity of the liposome membrane was found to have an effect on the penetration of tumors [61], and the rigidity could be finely tuned by adjusting the cholesterol concentration. Corosolic acid (CA) has a similar function as cholesterol in liposomes, but CA could be a constitutional component to construct stable liposomes. It has been previously reported that drug-loaded CA liposomes (CALP) not only have an anti-inflammatory effect, but also effectively fuse tumor cell membranes, thereby improving tumor permeability [289].
4.2. Exosomes
Exosomes are tiny EVs with a size of 30–150 nm and they are sealed with a lipid bilayer membrane. Compared with liposomes, exosomes have a more complex structure. A variety of drugs have been reported to be encapsulated in exosomes, including small drug molecules, nucleic acids and proteins. Exosomes can enter cells through three different mechanisms: endocytosis, membrane fusion with target cells, and internalization triggered by receptor-ligand interactions, which are shown in Fig. 13B. The surface proteins of exosomes or their lipid ligands with biological activity can bind to the surface receptors of recipient cells to realize signal transduction. The recipient cell can also engulf exosomes through endocytosis and the contents in the exosomes can be released in a controlled manner inside the recipient cell. In addition, exosomes can directly fuse with the recipient cell membrane, allowing them to deliver their contents into the recipient cell and achieve great control of drug release into target cells.
Multi-functional exosomes could be constructed from similar surface engineering techniques as liposomes. For example, multi-functional PMA/Au-BSA@Ce6 nanovehicles were synthesized from an amphiphilic polymer PMA. They penetrated tumors deeper because excessive singlet oxygen was produced from the nanovehicles under laser iradiation [290]. Tumor-exocytosed exosomes were supplied with AIE luminogen (AIEgen) to create hybrid nanovehicles [291], or biocompatible tumor-cell-exocytosed exosomes were used to create biomimetic porous silicon nanoparticles (PSiNPs) as a drug carrier to load DOX [292]. In order to improve drug penetration in solid tumors [15], exosomal surfaces were embellished with proteins that are membrane-bound in their native state.
Exosomes derived from plants and macrophages are recently attracting rising interest. The use of macrophage exosomes originated from their capacity to help transport drugs in the exosomes into the brain tissue, such as catalase [293] or curcumin [294]. The exosomes from natural macrophages were demonstrated to have good permeability for the delivery of protein drugs to the brain site [295]. To enhance tumor immunosuppression, tumor-associated macrophages (TAMs) could be treated to release exosomes with specific properties or function. For example, macrophage-derived microparticles that were treated with mannose (Man-MPs) were employed to load with metformin (Met@Man-MPs). This drug delivery system displayed beneficial effects in the H22 and 4T1 animal models [296]. PSiNPs, biomimetic porous silicon nanoparticles, were a very unique tumor-derived exosome. They were used as a nanomaterial delivery vehicle because they were highly effective against cancer in subcutaneous transplant tumors, in situ tumor models, and metastatic tumor models [295]. Exosomes secreted from human cells have been widely employed for drug delivery, while exosomes secreted by plants have been explored to construct nanovesicles for drug delivery. Due to a lower development cost, edible plant exosomes are emerging in the form of exosome-like nanovesicles (ELNVs) in the field of drug delivery. These plant-derived exosomes have relatively low oral immunogenicity, great tissue penetration, and excellent natural medicinal values [297,298]. Therefore, plant exosome-like nanovesicles (PELNVs) could be appealing as natural drug delivery systems compared to artificially made EVs.
4.3. Membrane coating technology for enhancing tumor penetration
After the encapsulation of nanoparticles by liposomes, the surface of their bilayer structures can be further coated with a mammalian cell membrane, thus promoting the internalization of liposomes-derived nanomedicine and overcoming extracellular barriers for antitumor drug delivery. For example, silica nanoparticle (MSN)-loaded liposomes were wrapped with MCF-7-derived cancer cell membranes (CCMs) to form a yolk-shell-structured nanomedicine (CCM@LM) [299]. CCM@LM successfully retained the membrane proteins of MCF-7 cells, and CCMs with these endogenous molecules on the surface prolong the circulation time of the nanomedicine, enhance its immune escape, and improve its targeting of tumor cells to a certain degree. It was illustrated that CCM@LM entered MCF-7 cells to release the encapsulated MSNs through a membrane fusion mechanism. The membrane surface on the CCM@LM was moderately rigid and it transformed into an elliptical shape during infiltration, thus facilitating effective tumor infiltration. This shape transformation of nanomedicine can be realized from after coating of erythrocytes membranes since they have greater deformability. For example, DOX-loaded, RBC membrane-coated elastic poly(ethylene glycol) diacrylate hydrogel nanoparticles (RBC-ENPs) [300], were compared with plain DOX-loaded PEGylated liposomes. RBC-ENPs showed a high diffusion rate, a high uptake level in tumors, deep inter-tumor penetration due to their RBC membrane deformability, thus obtaining effective antitumor effects. This RBC membrane coating technique could be extended to a variety of carriers, including drug-carrying liposomes.
The membrane of an exosome could be disassembled and applied to coat nanostructures. Exosome membrane (EM)-, cancer cell membrane- (CCM)-, and lipid-coated poly(lactic-co-glycolic acid) (PLGA) NPs (PLGA NPs) were prepared [301]. The results showed that EM-PLGA NPs exhibited better immune escape and more specific tumor targeting than CCM-PLGA NPs as well as lipid-PLGA NPs, thus achieving remarkable antitumor effects. In addition, there are highly active membrane-associated enzymes on the exosome membrane, and these membrane-bound proteins are in their natural state. The exosome membrane coating layer [15] could significantly strengthen the stability of the encapsulated nanoparticles and confer unique biological, physical or chemical properties to the resultant nanomedicine. The advances in membrane-coated technology [302] and exosome membrane extraction techniques could extend the potential of exosomes as a drug-carrying platform.
Finally, membrane-coated techniques are not only applicable to liposomes and exosomes, but also to the outer membrane derived from tumor cells, erythrocytes, leukocytes, platelets, simian viral membranes, and immune cells [[303], [304], [305], [306], [307]]. The design of the membrane shell using the CM-NPs concept can be tuned to improve stability and extend the retention time of the encapsulated drug in vivo [308], to overcome barriers in the tumor microenvironment [309], to enhance therapeutic effect of immunotherapy [271], or to enhance specific tumor targeting through membrane interaction [310] (Fig. 14). The membrane shell on the CM-NPs can be further functionalized [311], rendering the versatility of this membrane-coating technology.
Fig. 14.
Illustration of essential components of CM-NP. (Fig. 14 was created with BioRender.com.).
The membrane-coating technology for liposomes and exosomes can be extrapolated to chemically surface modification and functionalization of nanomedicine, so it becomes more stable during circulation as well as more efficient in intracellularization through membrane fusion to support deep penetration and anti-tumor effects of antitumor drugs in nanomedicine. Functional moieties or groups that can be attached to membranes have been summarized in great detail in review articles [312,313]. Such a comprehensive understanding will serve as a fundamental foundation for the innovative development of nanocarriers.
In practical application, it is critical to maintain the stability of cell-derived exosome membranes or functional groups/moieties on the surface of liposomes and exosomes. Contamination of cell-derived exosome membranes should be avoided to assure their safety. Although a few membranes have been applied for the membrane-coating technology, membranes derived from microorganisms, plants and animals could be explored to tailor the delivery of nanomedicine to specific tumor sites. In addition, chemically modified nanocarriers should be comprehensively evaluated in terms of their toxicity, compatibility, stability and functionality.
5. Future perspectives
Over the past two decades, the field of pharmaceutical nanotechnology has experienced remarkable growth and advancement. Nanomedicine becomes more effective, less toxic, and more intelligent than traditional drugs. This progress is attributed to the concerted efforts of academia and biopharmaceutical industry, resulting in successful development and clinical approval of a few nanomedicine drugs [314]. One notable example is BIND 014 [315], prepared from polymeric nanoparticles loaded with a cytotoxic agent docetaxel. It targets prostate membrane specific antigen (PMSA), and can effectively evade the immune system to reach the site of disease [316,317]. It has progressed into phase II clinical trials. While initial results are promising for prostate cancer, the trial fails when it is expanded to other cancer types such as head and neck and cervical. The failure may be ascribed to tumor heterogeneity, off-target effects, and insufficient tumor penetration. Thus it becomes critical to enhance tumor penetration of nanomedicine to effectively elevate the drug dose within all regions of solid tumors.
Tumor penetration is also crucial for solid tumor immunotherapy, as immune cell infiltration into tumors is in a broader form of therapeutic penetration. The distinctive properties between “hot” and “cold” tumors based on their immunogenicity have a significant impact on T cell infiltration. CAR-T cells for clinical use have been demonstrated with clinical success, while they face challenges such as a complex ex vivo production method, off-target toxicity, and tumor resistance. Nanomedicine in combination with CAR-T therapy [318] may improve the anti-tumor effect of genetically engineered T-cell therapy through an intrinsic delivery process. In addition, because not all patients respond well to anti-PD-L1 agents in immunotherapy due to inter-patient variabilities, removal of immunosuppressive cells by nanomedicine [319] may promote lymphocyte infiltration and amplify the effect of anti-PD-L1 agents. The combination of nanomedicine and immunotherapy could significantly enhance tumor penetration of infiltrated T cells, which opens a new door for the application of nanomedicine.
Challenges such as tumor heterogeneity and penetration of nanomedicine in large tumors remain significant barriers to clinical translation of nanomedicine. Impressive performance of nanomedicine in animal models often does not replicate in clinical trials, which could be due to distinctive discrepancies in tumor microenvironment and the immune system between animal models and patients. Evaluating the therapeutic performance of nanomedicine at the preclinical stage requires stable, reproducible animal models and advanced technical tools. The PDX model for preclinical stages may mitigate the discrepancies from tumor heterogeneity to some extend and it could be an appropriate model for assessment of nanomedicine penetration. Standardization of animal models and preclinical evaluation procedures is equally important for evaluating the penetration level of nanomedicine in solid tumors.
Tumor penetration of nanomedicine not only depends on chemical/physical/biological properties of nanomedicine, but also its interaction the tumor microenvironment. For example, the EPR effect is a fundamental principle in nanomedicine design and it is historically recognized as the primary factor for nanoparticle accumulation in tumors, while the EPR effect becomes insignificant in a clinical setting. Tumors do not exhibit a uniform EPR effect for the administrated nanomedicine, leading to inconsistent and unpredictable drug delivery results. Barriers such as a dense ECM and a high IFP in many tumors can pronouncedly hinder the penetration of nanomedicine even after it reaches the tumor site via the EPR effect.
Alternative nanoparticle transport mechanisms rather than the EPR effect have been explored by refining nanomedicine designs for targeting tumor cell organelles and realizing deep tumor penetration. One of the transport mechanisms is via active penetration strategies for nanomedicine, such as the intercellular transport, cationization-mediated transcytosis, and organelle-affinitive transfer. The organelle-affinitive transfer process by harnessing unique membrane properties of organelles such as the ER offers direct transfer of nanomedicine from the plasma membrane to the ER membrane, and bypass conventional endosome/lysosome pathways, holding great promise for overcoming tumor penetration barriers and addressing the challenges of tumor therapy.
As researchers gradually reveal the complex interplay between cellular structures, particularly key organelles such as the ER or mitochondria, the design of nanomedicine may be reshaped. This could herald the advent of nanomedicine that is not only more precise in targeting tumors, but also active in tumor penetration. These innovative approaches could potentially surmount the prevailing challenges in clinical translational development although the path from laboratory benches to clinical beds for nanomedicine remains laden with obstacles.
6. Conclusion
In general, therapeutic drugs for treating cancer must be delivered into the tumor cells, exert their biological function, and are eliminated from the body in the clinic application. Free drugs frequently fail to accumulate efficiently at the intended site, thus leading to toxic and adverse consequences. Nanoscale drug delivery systems with the EPR effect can help drug accumulation at the tumor site and reduce harmful and side effects of free drugs. However, deep penetration of drugs through these nanoscale carriers into solid tumors remains very challenging. Various strategies have been developed to enhance the penetration depth of drugs into the tumor, including (1) degrading the extracellular matrix through utilization of characteristic factors in the tumor cell microenvironment, such as matrix metalloproteinases, hypoxia-inducing factors, and tumor-associated fibroblasts, especially by targeting rich collagen and hyaluronic acid in the ECM; (2) Structural changes of the nanocarrier sequentially or spatially through responses to exogenous and endogenous stimuli; (3) An increase in the concentration of tiny chemicals like RNS and NO through physical means (e.g. ultrasound), especially improving effectiveness of nanoparticles penetrating the blood-brain barrier; (4) Surface engineering nano carriers, such as exosomes, liposomes, or cell-penetrating peptides.
To establish a unified standard in vivo or in vitro model that could completely map the solid tumors in the human body requires joint efforts from multidisciplinary collaboration. Mathematical modeling or machine learning through linear regression could be employed to achieve longitudinal or horizontal comparisons between different treatment groups or drugs. The outcomes of the anti-tumor effects could be assessed beyond survival rates and tumor sizes once the penetration model is standardized in time and space. Enhancing the drug penetration depth into tumors and elevating the accumulation of drugs in tumors eventually bring benefits to cancer patients with high efficacies and low adverse effects.
Ethics approval and consent to participate
The manuscript is a review article. The authors declare no experimentation on human or animals were designed.
CRediT authorship contribution statement
Xiaoding Shen: Conceptualization, Visualization, Writing – original draft. Dayi Pan: Conceptualization, Visualization, Writing – original draft, Funding acquisition. Qiyong Gong: Funding acquisition, Project administration, Supervision. Zhongwei Gu: Project administration, Supervision. Kui Luo: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Figs. 1, Fig. 2A, Fig. 3A–D, Figs. 4A, Fig. 6, Fig. 7B and C, Figs. 8, Fig. 11, Fig. 13, Fig. 14, and Graphical abstract were created with BioRender.com. This work was financially supported by the National Natural Science Foundation of China (32271445, 52073193, 32271382), National Key Research and Development Program of China (2022YFC2009900), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21013).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Advancing cancer therapy. Nat. Cancer. 2021;2:245–246. doi: 10.1038/s43018-021-00192-x. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Z., Li M. Targeted therapies for cancer. BMC Med. 2022;20(90) doi: 10.1186/s12916-022-02287-3. s12916-022-02287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quader S., Kataoka K. Nanomaterial-enabled cancer therapy. Mol. Ther. 2017;25:1501–1513. doi: 10.1016/j.ymthe.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan D., Cao Y., Cao M., Wang Y., Cao Y., Gong T. Nanomedicine in cancer therapy. Signal Transduction Targeted Ther. 2023;8:293. doi: 10.1038/s41392-023-01536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duangjai A., Luo K., Zhou Y., Yang J., Kopeček J. Combination cytotoxicity of backbone degradable HPMA copolymer gemcitabine and platinum conjugates toward human ovarian carcinoma cells. Eur. J. Pharm. Biopharm. 2014;87:187–196. doi: 10.1016/j.ejpb.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giraldo N.A., Sanchez Salas R., Peske J.D., Vano Y., Becht E., Petitprez F., Validire P., Ingels A., Cathelineau X., Fridman W.H., Sautès Fridman C. The clinical role of the TME in solid cancer. Br. J. Cancer. 2019;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson N.M., Simon M.C. The tumor microenvironment. Curr. Biol. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H., Wang Y., Bao L., Chen C. Engineering nano-bio interfaces from nanomaterials to nanomedicines. Acc. Mater. Res. 2022;3:812–829. doi: 10.1021/accountsmr.2c00072. [DOI] [Google Scholar]
- 9.Li Z., Shan X., Chen Z., Gao N., Zeng W., Zeng X., Mei L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv. Sci. 2021;8 doi: 10.1002/advs.202002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Zhang J., Gao Y., Jiang Y., Guan Z., Xie Y., Hu J., Chen J. Barrier permeation and improved nanomedicine delivery in tumor microenvironments. Cancer Lett. 2023;562 doi: 10.1016/j.canlet.2023.216166. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Lin R., Li H., He W., Du J., Wang J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. WIREs Nanomed. Nanobiotechnol. 2019;11:e1519. doi: 10.1002/wnan.1519. [DOI] [PubMed] [Google Scholar]
- 12.Wang H.X., Zuo Z.Q., Du J.Z., Wang Y.C., Sun R., Cao Z.T., Ye X.D., Wang J.L., Leong K.W., Wang J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today. 2016;11:133–144. doi: 10.1016/j.nantod.2016.04.008. [DOI] [Google Scholar]
- 13.Wang R., Yin C., Liu C., Sun Y., Xiao P., Li J., Yang S., Wu W., Jiang X. Phenylboronic acid modification augments the lysosome escape and antitumor efficacy of a cylindrical polymer brush-based prodrug. J. Am. Chem. Soc. 2021;143:20927–20938. doi: 10.1021/jacs.1c09741. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Wu J., Hu X., Ding T., Tang T., Xiang D. Biomimetic liposome with surface-bound elastase for enhanced tumor penetration and chemo-immumotherapy. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202100794. [DOI] [PubMed] [Google Scholar]
- 15.Hong Y., Nam G.H., Koh E., Jeon S., Kim G.B., Jeong C., Kim D.H., Yang Y., Kim I.S. Exosome as a vehicle for delivery of membrane protein therapeutics, PH20, for enhanced tumor penetration and antitumor efficacy. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201703074. [DOI] [Google Scholar]
- 16.Barattin M., Mattarei A., Balasso A., Paradisi C., Cantù L., Del Favero E., Viitala T., Mastrotto F., Caliceti P., Salmaso S. pH-controlled liposomes for enhanced cell penetration in tumor environment. ACS Appl. Mater. Interfaces. 2018;10:17646–17661. doi: 10.1021/acsami.8b03469. [DOI] [PubMed] [Google Scholar]
- 17.He X., Chen X., Liu L., Zhang Y., Lu Y., Zhang Y., Chen Q., Ruan C., Guo Q., Li C., Sun T., Jiang C. Sequentially triggered nanoparticles with tumor penetration and intelligent drug release for pancreatic cancer therapy. Adv. Sci. 2018;5 doi: 10.1002/advs.201701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu D., Duo Y., Suo M., Zhao Y., Xia L., Zheng Z., Li Y., Tang B.Z. Tumor-exocytosed exosome/aggregation-induced emission luminogen hybrid nanovesicles facilitate efficient tumor penetration and photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:13836–13843. doi: 10.1002/anie.202003672. [DOI] [PubMed] [Google Scholar]
- 19.Bilotta M.T., Antignani A., Fitzgerald D.J. Managing the TME to improve the efficacy of cancer therapy. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.954992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks K., Ohms S., Van Zijl P., Denny W., Hunter P., Wilson W. An experimental and mathematical model for the extravascular transport of a DNA intercalator in tumours. Br. J. Cancer. 1997;76:894–903. doi: 10.1038/bjc.1997.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J., Sun J., Liu Y., Chong G., Li Y., Dong H. Nanosystem-mediated lactate modulation in the tumor micro environment for enhanced cancer therapy. Nano Res. 2023;16:654–671. doi: 10.1007/s12274-022-4620-z. [DOI] [Google Scholar]
- 22.Hicks K., Ohms S., Van Zijl P., Denny W., Hunter P., Wilson W. An experimental and mathematical model for the extravascular transport of a DNA intercalator in tumours. Br. J. Cancer. 1997;76:894–903. doi: 10.1038/bjc.1997.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grantab R., Sivananthan S., Tannock I.F. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 2006;66:1033–1039. doi: 10.1158/0008-5472.CAN-05-3077. [DOI] [PubMed] [Google Scholar]
- 24.Al Abd A.M., Lee J.H., Kim S.Y., Kun N., Kuh H.J. Novel application of multicellular layers culture for in situ evaluation of cytotoxicity and penetration of paclitaxel. Cancer Sci. 2008;99:423–431. doi: 10.1111/j.1349-7006.2007.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunggal J.K., Melo T., Ballinger J.R., Tannock I.F. The influence of expression of P-glycoprotein on the penetration of anticancer drugs through multicellular layers. Int. J. Cancer. 2000;86:101–107. doi: 10.1002/(SICI)1097-0215(20000401)86:1<101::AID-IJC16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Cowan D.S.M., Tannock I.F. Factors that influence the penetration of methotrexate through solid tissue. Int. J. Cancer. 2001;91:120–125. doi: 10.1002/1097-0215(20010101)91:1<120::AID-IJC1021>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.M.S. Choi, S.H. Kim, H.J.Kuh, Penetration of paclitaxel and 5-fluorouracil in multicellular layers of human colorectal cancer cells, Oncol. Rep. 25 (2011) 863–870, doi:10.3892/or.2011.1138. [DOI] [PubMed]
- 28.Yohan D., Cruje C., Lu X., Chithrani D. Elucidating the uptake and distribution of nanoparticles in solid tumors via a multilayered cell culture model. Nano-Micro Lett. 2015;7:127–137. doi: 10.1007/s40820-014-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G., Zhang C., Jiang Y., Song Y., Chen J., Sun Y., Li Q., Zhou Z., Shen Y., Huang P. Ultrasonic cavitation-assisted and acid-activated transcytosis of liposomes for universal active tumor penetration. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202102786. [DOI] [Google Scholar]
- 30.Peng J., Chen F., Liu Y., Zhang F., Cao L., You Q., Yang D., Chang Z., Ge M., Li L., Wang Z., Mei Q., Shao D., Chen M., Dong W.F. A light-driven dual-nanotransformer with deep tumor penetration for efficient chemo-immunotherapy. Theranostics. 2022;12:1756–1768. doi: 10.7150/thno.68756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G., Wu B., Li Q., Chen S., Jin X., Liu Y., Zhou Z., Shen Y., Huang P. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small. 2020;16 doi: 10.1002/smll.202004172. [DOI] [PubMed] [Google Scholar]
- 32.Jensen C., Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., Ibbs M., Bliźniak R., Łuczewski Ł., Lamperska K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016;4:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ando Y., Siegler E.L., Ta H.P., Cinay G.E., Zhou H., Gorrell K.A., Au H., Jarvis B.M., Wang P., Shen K. Evaluating CAR-T cell therapy in a hypoxic 3D tumor model. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Świerczewska M. The response and resistance to drugs in ovarian cancer cell lines in 2D monolayers and 3D spheroids. Biomed. Pharmacother. 165. 2023:115152. doi: 10.1016/j.biopha.2023.115152. [DOI] [PubMed] [Google Scholar]
- 36.Fontoura J.C., Viezzer C., Dos Santos F.G., Ligabue R.A., Weinlich R., Puga R.D., Antonow D., Severino P., Bonorino C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng., C. 2020;107 doi: 10.1016/j.msec.2019.110264. [DOI] [PubMed] [Google Scholar]
- 37.Xue X., Gao Q. Establishment and validation of clinical prediction model in WHO grade II glioma. Neuro Oncol. 2021;23:ii28. doi: 10.1093/neuonc/noab180.096. [DOI] [Google Scholar]
- 38.Ermis M., Falcone N., Roberto De Barros N., Mecwan M., Haghniaz R., Choroomi A., Monirizad M., Lee Y., Song J., Cho H.J., Zhu Y., Kang H., Dokmeci M.R., Khademhosseini A., Lee J., Kim H.J. Tunable hybrid hydrogels with multicellular spheroids for modeling desmoplastic pancreatic cancer. Bioact. Mater. 2023;25:360–373. doi: 10.1016/j.bioactmat.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh A., Tayalia P. Three-dimensional cryogel matrix for spheroid formation and anti-cancer drug screening. J. Biomed. Mater. Res., Part A. 2020;108:365–376. doi: 10.1002/jbm.a.36822. [DOI] [PubMed] [Google Scholar]
- 40.Truong D.D., Kratz A., Park J.G., Barrientos E.S., Saini H., Nguyen T., Pockaj B., Mouneimne G., LaBaer J., Nikkhah M. A human organotypic microfluidic tumor model permits investigation of the interplay between patient-derived fibroblasts and breast cancer cells. Cancer Res. 2019;79:3139–3151. doi: 10.1158/0008-5472.CAN-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su G., Zhao Y., Wei J., Han J., Chen L., Xiao Z., Chen B., Dai J. The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials. 2013;34:3215–3222. doi: 10.1016/j.biomaterials.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 42.Zheng X.L., Pan D.Y., Zhu G.N., Zhang L., Bhamra A., Chen R.J., Zhang H., Gong Q.Y., Gu Z.W., Luo K. A dendritic polymer-based nanosystem mediates drug penetration and irreversible endoplasmic reticulum stresses in tumor via neighboring effect. Adv. Mater. 2022;34 doi: 10.1002/adma.202201200. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Chen M., Yao B., Lu X., Song B., Vasilatos S.N., Zhang X., Ren X., Yao C., Bian W., Sun L. Dual pH/ROS-responsive nanoplatform with deep tumor penetration and self-amplified drug release for enhancing tumor chemotherapeutic efficacy. Small. 2020;16 doi: 10.1002/smll.202002188. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Chen W., Yang C., Fan Q., Wu W., Jiang X. Enhancing tumor penetration and targeting using size-minimized and zwitterionic nanomedicines. J. Control. Release. 2016;237:115–124. doi: 10.1016/j.jconrel.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Zeng X., Li P., Yan S., Liu B.F. Reduction/pH-responsive disassemblable MOF-microbial nanohybrid for targeted tumor penetration and synergistic therapy. Chem. Eng. J. 2023;452 doi: 10.1016/j.cej.2022.139517. [DOI] [Google Scholar]
- 46.Zhou F., Feng B., Wang T., Wang D., Meng Q., Zeng J., Zhang Z., Wang S., Yu H., Li Y. Programmed multiresponsive vesicles for enhanced tumor penetration and combination therapy of triple-negative breast cancer. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201606530. [DOI] [Google Scholar]
- 47.Xiong X., Xu Z., Huang H., Wang Y., Zhao J., Guo X., Zhou S. A NIR light triggered disintegratable nanoplatform for enhanced penetration and chemotherapy in deep tumor tissues. Biomaterials. 2020;245 doi: 10.1016/j.biomaterials.2020.119840. [DOI] [PubMed] [Google Scholar]
- 48.Yao H., Guo X., Zhou H., Ren J., Li Y., Duan S., Gong X., Du B. Mild acid-responsive “nanoenzyme capsule” remodeling of the tumor microenvironment to increase tumor penetration. ACS Appl. Mater. Interfaces. 2020;12:20214–20227. doi: 10.1021/acsami.0c03022. [DOI] [PubMed] [Google Scholar]
- 49.Huo M., Zhou J., Wang H., Zheng Y., Tong Y., Zhou J., Liu J., Yin T. A pHe sensitive nanodrug for collaborative penetration and inhibition of metastatic tumors. J. Control. Release. 2022;352:893–908. doi: 10.1016/j.jconrel.2022.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Xiao X., Cai H., Huang Q., Wang B., Wang X., Luo Q., Li Y., Zhang H., Gong Q., Ma X., Gu Z., Luo K. Polymeric dual-modal imaging nanoprobe with two-photon aggregation-induced emission for fluorescence imaging and gadolinium-chelation for magnetic resonance imaging. Bioact. Mater. 2023;19:538–549. doi: 10.1016/j.bioactmat.2022.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Z., Wang T., Wang J., Xu J., Shen T., Zhang T., Zhang B., Gao S., Zhao C., Yang M., Sheng F., Yu J., Hou Y. Self-propelled janus nanocatalytic robots guided by magnetic resonance imaging for enhanced tumor penetration and therapy. J. Am. Chem. Soc. 2023;145:11019–11032. doi: 10.1021/jacs.2c12219. [DOI] [PubMed] [Google Scholar]
- 52.Li H.N., Sun J.Y., Zhu H.Y., Wu H.X., Zhang H., Gu Z.W., Luo K. Recent advances in development of dendritic polymer-based nanomedicines for cancer diagnosis. WIREs Nanomed. Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1670. [DOI] [PubMed] [Google Scholar]
- 53.Yao D., Wang Y., Zou R., Bian K., Liu P., Shen S., Yang W., Zhang B., Wang D. Molecular engineered squaraine nanoprobe for NIR-II/photoacoustic imaging and photothermal therapy of metastatic breast cancer. ACS Appl. Mater. Interfaces. 2020;12:4276–4284. doi: 10.1021/acsami.9b20147. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y., Song Y., Cao Z., Dong L., Lu Y., Yang X., Wang J. Magnetically actuated active deep tumor penetration of deformable large nanocarriers for enhanced cancer therapy. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202103655. [DOI] [Google Scholar]
- 55.Chen S., Zhong Y., Fan W., Xiang J., Wang G., Zhou Q., Wang J., Geng Y., Sun R., Zhang Z., Piao Y., Wang J., Zhuo J., Cong H., Jiang H., Ling J., Li Z., Yang D., Yao X., Xu X., Zhou Z., Tang J., Shen Y. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 2021;5:1019–1037. doi: 10.1038/s41551-021-00701-4. [DOI] [PubMed] [Google Scholar]
- 56.Wang G., Zhou Z., Zhao Z., Li Q., Wu Y., Yan S., Shen Y., Huang P. Enzyme-triggered transcytosis of dendrimer-drug conjugate for deep penetration into pancreatic tumors. ACS Nano. 2020;14:4890–4904. doi: 10.1021/acsnano.0c00974. [DOI] [PubMed] [Google Scholar]
- 57.Yan J., Wu Q., Zhao Z., Wu J., Ye H., Liang Q., Zhou Z., Hou M., Li X., Liu Y., Yin L. Light-assisted hierarchical intratumoral penetration and programmed antitumor therapy based on tumor microenvironment (TME)-amendatory and self-adaptive polymeric nanoclusters. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120166. [DOI] [PubMed] [Google Scholar]
- 58.Zhou M., Huang H., Wang D., Lu H., Chen J., Chai Z., Yao S.Q., Hu Y. Light-triggered PEGylation/dePEGylation of the nanocarriers for enhanced tumor penetration. Nano Lett. 2019;19:3671–3675. doi: 10.1021/acs.nanolett.9b00737. [DOI] [PubMed] [Google Scholar]
- 59.An H.W., Li L.L., Wang Y., Wang Z., Hou D., Lin Y.X., Qiao S.L., Wang M.D., Yang C., Cong Y., Ma Y., Zhao X.X., Cai Q., Chen W.T., Lu C.Q., Xu W., Wang H., Zhao Y. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nat. Commun. 2019;10:4861. doi: 10.1038/s41467-019-12848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan J., Zhu R., Wu F., Zhao Z., Ye H., Hou M., Liu Y., Yin L. iRGD-reinforced, photo-transformable nanoclusters toward cooperative enhancement of intratumoral penetration and antitumor efficacy. Nano Res. 2020;13:2706–2715. doi: 10.1007/s12274-020-2913-7. [DOI] [Google Scholar]
- 61.Wu H., Yu M., Miao Y., He S., Dai Z., Song W., Liu Y., Song S., Ahmad E., Wang D., Gan Y. Cholesterol-tuned liposomal membrane rigidity directs tumor penetration and anti-tumor effect. Acta Pharm. Sin. B. 2019;9:858–870. doi: 10.1016/j.apsb.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ji T., Lang J., Wang J., Cai R., Zhang Y., Qi F., Zhang L., Zhao X., Wu W., Hao J., Qin Z., Zhao Y., Nie G. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy. ACS Nano. 2017;11:8668–8678. doi: 10.1021/acsnano.7b01026. [DOI] [PubMed] [Google Scholar]
- 63.He Y., Tian X., Fan X., Gong X., Tan S., Pan A., Liang S., Xu H., Zhou F. Enzyme-triggered size-switchable nanosystem for deep tumor penetration and hydrogen therapy. ACS Appl. Mater. Interfaces. 2023;15:552–565. doi: 10.1021/acsami.2c18184. [DOI] [PubMed] [Google Scholar]
- 64.Ma T., Chen R., Lv N., Chen Y., Qin H., Jiang H., Zhu J. Size-transformable bicomponent peptide nanoparticles for deep tumor penetration and photo-chemo combined antitumor therapy. Small. 2022;18 doi: 10.1002/smll.202106291. [DOI] [PubMed] [Google Scholar]
- 65.Zhu X., Li C., Lu Y., Liu Y., Wan D., Zhu D., Pan J., Ma G. Tumor microenvironment-activated therapeutic peptide-conjugated prodrug nanoparticles for enhanced tumor penetration and local T cell activation in the tumor microenvironment. Acta Biomater. 2021;119:337–348. doi: 10.1016/j.actbio.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Wong C., Stylianopoulos T., Cui J., Martin J., Chauhan V.P., Jiang W., Popović Z., Jain R.K., Bawendi M.G., Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dane E.L., Belessiotis Richards A., Backlund C., Wang J., Hidaka K., Milling L.E., Bhagchandani S., Melo M.B., Wu S., Li N., Donahue N., Ni K., Ma L., Okaniwa M., Stevens M.M., Alexander Katz A., Irvine D.J. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 2022;21:710–720. doi: 10.1038/s41563-022-01251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong S.M., Costa D.F., Jagielska A., Van Vliet K.J., Hammond P.T. Stiffness of targeted layer-by-layer nanoparticles impacts elimination half-life, tumor accumulation, and tumor penetration. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2104826118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seo J.W., Fu K.Y., Correa S., Eisenstein M., Appel E.A., Soh H.T. Real-time monitoring of drug pharmacokinetics within tumor tissue in live animals. Sci. Adv. 2022;8:eabk2901. doi: 10.1126/sciadv.abk2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thurber G.M., Schmidt M.M., Wittrup K.D. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chauhan V.P., Stylianopoulos T., Martin J.D., Popović Z., Chen O., Kamoun W.S., Bawendi M.G., Fukumura D., Jain R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae I.Y., Choi W., Oh S.J., Kim C., Kim S. TIMP -1-expressing breast tumor spheroids for the evaluation of drug penetration and efficacy. Bioeng. Transl. Med. 2022;7 doi: 10.1002/btm2.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H., Zhou L., Xie K., Wu J., Song P., Xie H., Zhou L., Liu J., Xu X., Shen Y., Zheng S. Polylactide-tethered prodrugs in polymeric nanoparticles as reliable nanomedicines for the efficient eradication of patient-derived hepatocellular carcinoma. Theranostics. 2018;8:3949–3963. doi: 10.7150/thno.26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q.J., Liao Z.Y., Han L., Li L.Y., Song Y., Song E.Q. Isolation and analysis of tumor cell subpopulations using biomimetic immuno-fluorescent. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202004963. [DOI] [Google Scholar]
- 75.Li W., Zhou Z., Zhou X., Khoo B.L., Gunawan R., Chin Y.R., Zhang L., Yi C., Guan X., Yang M. 3D biomimetic models to reconstitute tumor microenvironment in vitro: spheroids, organoids, and tumor-on-a-chip. Adv. Healthcare Mater. 2023;12 doi: 10.1002/adhm.202202609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Portman N., Lim E. A new sophistication for breast cancer PDXs. Nat. Cancer. 2022;3:138–140. doi: 10.1038/s43018-021-00328-z. [DOI] [PubMed] [Google Scholar]
- 77.Tan P., Cai H., Wei Q., Tang X., Zhang Q., Kopytynski M., Yang J., Yi Y., Zhang H., Gong Q., Gu Z., Chen R., Luo K. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121061. [DOI] [PubMed] [Google Scholar]
- 78.Yan W., Guo B., Wang Z., Yang J., Zhong Z., Meng F. RGD-directed 24 nm micellar docetaxel enables elevated tumor-liver ratio, deep tumor penetration and potent suppression of solid tumors. J. Control. Release. 2023;360:304–315. doi: 10.1016/j.jconrel.2023.06.032. [DOI] [PubMed] [Google Scholar]
- 79.Wu S., Wang J., Fu Z., Familiari G., Relucenti M., Aschner M., Li X., Chen H., Chen R. Matairesinol nanoparticles restore chemosensitivity and suppress colorectal cancer progression in preclinical models: role of lipid metabolism reprogramming. Nano Lett. 2023;23:1970–1980. doi: 10.1021/acs.nanolett.3c00035. [DOI] [PubMed] [Google Scholar]
- 80.Bouffi C., Wikenheiser Brokamp K.A., Chaturvedi P., Sundaram N., Goddard G.R., Wunderlich M., Brown N.E., Staab J.F., Latanich R., Zachos N.C., Holloway E.M., Mahe M.M., Poling H.M., Vales S., Fisher G.W., Spence J.R., Mulloy J.C., Zorn A.M., Wells J.M., Helmrath M.A. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat. Biotechnol. 2023;41:824–831. doi: 10.1038/s41587-022-01558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allen T.M., Brehm M.A., Bridges S., Ferguson S., Kumar P., Mirochnitchenko O., Palucka K., Pelanda R., Sanders Beer B., Shultz L.D., Su L., PrabhuDas M. Humanized immune system mouse models: progress, challenges and opportunities. Nat. Immunol. 2019;20:770–774. doi: 10.1038/s41590-019-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das R., Strowig T., Verma R., Koduru S., Hafemann A., Hopf S., Kocoglu M.H., Borsotti C., Zhang L., Branagan A., Eynon E., Manz M.G., Flavell R.A., Dhodapkar M.V. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat. Med. 2016;22:1351–1357. doi: 10.1038/nm.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujiwara S. Humanized mice: a brief overview on their diverse applications in biomedical research. J. Cell. Physiol. 2018;233:2889–2901. doi: 10.1002/jcp.26022. [DOI] [PubMed] [Google Scholar]
- 84.Lai Y., Wei X., Lin S., Qin L., Cheng L., Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J. Hematol. Oncol. 2017;10:106. doi: 10.1186/s13045-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rios Doria J., Stevens C., Maddage C., Lasky K., Koblish H.K. Characterization of human cancer xenografts in humanized mice. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De La Rochere P., Guil Luna S., Decaudin D., Azar G., Sidhu S.S., Piaggio E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39:748–763. doi: 10.1016/j.it.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Prokoph N., Matthews J.D., Trigg R.M., Montes Mojarro I.A., Burke G.A.A., Fend F., Merkel O., Kenner L., Geoerger B., Johnston R., Murray M.J., Riguad C., Brugières L., Turner S.D. Patient-derived xenograft models of ALK + ALCL reveal preclinical promise for therapy with brigatinib. Br. J. Haematol. 2023;202:985–994. doi: 10.1111/bjh.18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou J., Neff C.P., Liu X., Zhang J., Li H., Smith D.D., Swiderski P., Aboellail T., Huang Y., Du Q., Liang Z., Peng L., Akkina R., Rossi J.J. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011;19:2228–2238. doi: 10.1038/mt.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malhi H., Homad L.J., Wan Y.H., Poudel B., Fiala B., Borst A.J., Wang J.Y., Walkey C., Price J., Wall A., Singh S., Moodie Z., Carter L., Handa S., Correnti C.E., Stoddard B.L., Veesler D., Pancera M., Olson J., King N.P., McGuire A.T. Immunization with a self-assembling nanoparticle vaccine displaying EBV gH/gL protects humanized mice against lethal viral challenge. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Theocharides A.P.A., Rongvaux A., Fritsch K., Flavell R.A., Manz M.G. Humanized hemato-lymphoid system mice. Haematologica. 2016;101:5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borodovsky A., McQuiston T.J., Stetson D., Ahmed A., Whitston D., Zhang J., Grondine M., Lawson D., Challberg S.S., Zinda M., Pollok B.A., Dougherty B.A., D'Cruz C.M. Generation of stable PDX derived cell lines using conditional reprogramming. Mol. Cancer. 2017;16:177. doi: 10.1186/s12943-017-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhong M., Fu L. Culture and application of conditionally reprogrammed primary tumor cells. Gastroenterol. Rep. 2020;8:224–233. doi: 10.1093/gastro/goaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun J., Chen Y., Xu J., Song X., Wan Z., Du Y., Ma W., Li X., Zhang L., Li S. High loading of hydrophobic and hydrophilic agents via small immunostimulatory carrier for enhanced tumor penetration and combinational therapy. Theranostics. 2020;10:1136–1150. doi: 10.7150/thno.38287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Y., Xiong J., Sun X., Gao H. Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Acta Pharm. Sin. B. 2022;12:4327–4347. doi: 10.1016/j.apsb.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shan Y., Ni Q., Zhang Q., Zhang M., Wei B., Cheng L., Zhong C., Wang X., Wang Q., Liu J., Zhang J., Wu J., Wang G., Zhou F. Targeting tumor endothelial hyperglycolysis enhances immunotherapy through remodeling tumor microenvironment. Acta Pharm. Sin. B. 2022;12:1825–1839. doi: 10.1016/j.apsb.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao Y., Ge X., Wei Y., He L., Wu A., Li J. Tumor microenvironment-activatable neuropeptide-drug conjugates enhanced tumor penetration and inhibition via multiple delivery pathways and calcium deposition. Chin. Chem. Lett. 2023 doi: 10.1016/j.cclet.2023.108672. [DOI] [Google Scholar]
- 97.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo Z., Hu Y., Zhao M., Hao K., He P., Tian H., Chen X., Chen M. Prodrug-based versatile nanomedicine with simultaneous physical and physiological tumor penetration for enhanced cancer chemo-immunotherapy. Nano Lett. 2021;21:3721–3730. doi: 10.1021/acs.nanolett.0c04772. [DOI] [PubMed] [Google Scholar]
- 99.Cox T.R. The matrix in cancer. Nat. Rev. Cancer. 2021;21:217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 100.Bellomo C., Caja L., Moustakas A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer. 2016;115:761–769. doi: 10.1038/bjc.2016.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 102.Sasaki K., Ishihara J., Ishihara A., Miura R., Mansurov A., Fukunaga K., Hubbell J.A. Engineered collagen-binding serum albumin as a drug conjugate carrier for cancer therapy. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw6081. eaaw6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhushan B., Khanadeev V., Khlebtsov B., Khlebtsov N., Gopinath P. Impact of albumin based approaches in nanomedicine: imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017;246:13–39. doi: 10.1016/j.cis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 104.Kolosnjaj Tabi J., Marangon I., Nicolas Boluda A., Silva A.K.A., Gazeau F. Nanoparticle-based hyperthermia, a local treatment modulating the tumor extracellular matrix. Pharmacol. Res. 2017;126:123–137. doi: 10.1016/j.phrs.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Liu Y., Gao X., Li X., Niu X., Yuan Z., Wang W. Near-infrared-light induced nanoparticles with enhanced tumor tissue penetration and intelligent drug release. Acta Biomater. 2019;90:314–323. doi: 10.1016/j.actbio.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 106.Xu F., Huang X., Wang Y., Zhou S. A size-changeable collagenase-modified nanoscavenger for increasing penetration and retention of nanomedicine in deep tumor tissue. Adv. Mater. 2020;32 doi: 10.1002/adma.201906745. [DOI] [PubMed] [Google Scholar]
- 107.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li G., Fan Y., Lin L., Wu R., Shen M., Shi X. Two-dimensional LDH nanodisks modified with hyaluronidase enable enhanced tumor penetration and augmented chemotherapy. Sci. China Chem. 2021;64:817–826. doi: 10.1007/s11426-020-9933-4. [DOI] [Google Scholar]
- 109.Hynes R.O., Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a004903. a004903-a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahalanobish S., Saha S., Dutta S., Sil P.C. Matrix metalloproteinase: an upcoming therapeutic approach for idiopathic pulmonary fibrosis. Pharmacol. Res. 2020;152 doi: 10.1016/j.phrs.2019.104591. [DOI] [PubMed] [Google Scholar]
- 111.Shu M., Tang J., Chen L., Zeng Q., Li C., Xiao S., Jiang Z., Liu J. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120574. [DOI] [PubMed] [Google Scholar]
- 112.Jia W., Liu R., Wang Y., Hu C., Yu W., Zhou Y., Wang L., Zhang M., Gao H., Gao X. Dual-responsive nanoparticles with transformable shape and reversible charge for amplified chemo-photodynamic therapy of breast cancer. Acta Pharm. Sin. B. 2022;12:3354–3366. doi: 10.1016/j.apsb.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao Q., Choi J.H., Dai Z., Wang J., Kim D., Tang X., Zhu L. Improving tumor specificity and anticancer activity of dasatinib by dual-targeted polymeric micelles. ACS Appl. Mater. Interfaces. 2017;9:36642–36654. doi: 10.1021/acsami.7b12233. [DOI] [PubMed] [Google Scholar]
- 114.Zhu L., Wang T., Perche F., Taigind A., Torchilin V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA. 2013;110:17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Z., Guo J., Sun J., Liang P., Wei Y., Deng X., Gao W. Thermoresponsive and protease-cleavable interferon-polypeptide conjugates with spatiotemporally programmed two-step release kinetics for tumor therapy. Adv. Sci. 2019;6 doi: 10.1002/advs.201900586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei Y., Song S., Duan N., Wang F., Wang Y., Yang Y., Peng C., Li J., Nie D., Zhang X., Guo S., Zhu C., Yu M., Gan Y. MT1-MMP-activated liposomes to improve tumor blood perfusion and drug delivery for enhanced pancreatic cancer therapy. Adv. Sci. 2020;7 doi: 10.1002/advs.201902746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou J., Yin Q., Li S., Yang R., Lou R., Sun Y., Du B. A deep tumor penetration nanoplatform for glycolysis inhibition and antimetastasis of breast cancer. J. Mater. Chem. B. 2022;10:4306–4320. doi: 10.1039/D1TB01759D. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y.X., Zhou J., Chen X.T., Li Z.Q., Gu L., Pan D.Y., Zheng X.L., Zhang Q.D., Chen R.J., Zhang H., Gong Q.Y., Gu Z.W., Luo K. Modulating tumor-stromal crosstalk via a redox-responsive nanomedicine for combination tumor therapy. J. Control. Release. 2023;356:525–541. doi: 10.1016/j.jconrel.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 119.Salavati H., Debbaut C., Pullens P., Ceelen W. Interstitial fluid pressure as an emerging biomarker in solid tumors. Biochim. Biophys. Acta, Rev. Cancer. 2022;1877 doi: 10.1016/j.bbcan.2022.188792. [DOI] [PubMed] [Google Scholar]
- 120.Yan J., Wu Q., Zhao Z., Wu J., Ye H., Liang Q., Zhou Z., Hou M., Li X., Liu Y., Yin L. Light-assisted hierarchical intratumoral penetration and programmed antitumor therapy based on tumor microenvironment (TME)-amendatory and self-adaptive polymeric nanoclusters. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120166. [DOI] [PubMed] [Google Scholar]
- 121.Pang N., Li J., Sun A., Yang Z., Cheng S., Qi X.R. Prior anti-CAFs break down the CAFs barrier and improve accumulation of docetaxel micelles in tumor. Int. J. Nanomed. 2018;13:5971–5990. doi: 10.2147/IJN.S171224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Godet I., Shin Y.J., Ju J.A., Ye I.C., Wang G., Gilkes D.M. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 2019;10:4862. doi: 10.1038/s41467-019-12412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jain R.K. Antiangiogenesis Strategies Revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khawar I.A., Kim J.H., Kuh H.J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Control. Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 125.Eisinger Mathason T.S.K., Zhang M., Qiu Q., Skuli N., Nakazawa M.S., Karakasheva T., Mucaj V., Shay J.E.S., Stangenberg L., Sadri N., Puré E., Yoon S.S., Kirsch D.G., Simon M.C. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y., Wang J., Li H., Guo M., Sun X., Liu C., Yu C. MnO2 decorated metal-organic framework-based hydrogel relieving tumor hypoxia for enhanced photodynamic therapy. Macromol. Rapid Commun. 2023 doi: 10.1002/marc.202300268. [DOI] [PubMed] [Google Scholar]
- 127.Wu X., Zhu Y., Huang W., Li J., Zhang B., Li Z., Yang X. Hyperbaric oxygen potentiates doxil antitumor efficacy by promoting tumor penetration and sensitizing cancer cells. Adv. Sci. 2018;5 doi: 10.1002/advs.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen H., Bi Q., Yao Y., Tan N. Dimeric BODIPY-loaded liposomes for dual hypoxia marker imaging and activatable photodynamic therapy against tumors. J. Mater. Chem. B. 2018;6:4351–4359. doi: 10.1039/C8TB00665B. [DOI] [PubMed] [Google Scholar]
- 129.Taylor C.T., Scholz C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022;18:573–587. doi: 10.1038/s41581-022-00587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McGettrick A.F., O’Neill L.A.J. The role of HIF in immunity and inflammation. Cell Metab. 2020;32:524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 131.Ishihara J., Ishihara A., Sasaki K., Lee S.S.Y., Williford J.M., Yasui M., Abe H., Potin L., Hosseinchi P., Fukunaga K., Raczy M.M., Gray L.T., Mansurov A., Katsumata K., Fukayama M., Kron S.J., Swartz M.A., Hubbell J.A. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau3259. eaau3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mansurov A., Ishihara J., Hosseinchi P., Potin L., Marchell T.M., Ishihara A., Williford J.M., Alpar A.T., Raczy M.M., Gray L.T., Swartz M.A., Hubbell J.A. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 2020;4:531–543. doi: 10.1038/s41551-020-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zinger A., Koren L., Adir O., Poley M., Alyan M., Yaari Z., Noor N., Krinsky N., Simon A., Gibori H., Krayem M., Mumblat Y., Kasten S., Ofir S., Fridman E., Milman N., Lübtow M.M., Liba L., Shklover J., Shainsky Roitman J., Binenbaum Y., Hershkovitz D., Gil Z., Dvir T., Luxenhofer R., Satchi Fainaro R., Schroeder A. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13:11008–11021. doi: 10.1021/acsnano.9b02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X., Luo J., He L., Cheng X., Yan G., Wang J., Tang R. Hybrid pH-sensitive nanogels surface-functionalized with collagenase for enhanced tumor penetration. J. Colloid Interface Sci. 2018;525:269–281. doi: 10.1016/j.jcis.2018.04.084. [DOI] [PubMed] [Google Scholar]
- 135.Ikeda Imafuku M., Gao Y., Shaha S., Wang L.L.W., Park K.S., Nakajima M., Adebowale O., Mitragotri S. Extracellular matrix degrading enzyme with stroma-targeting peptides enhance the penetration of liposomes into tumors. J. Control. Release. 2022;352:1093–1103. doi: 10.1016/j.jconrel.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 136.Andreone B.J., Lacoste B., Gu C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 2015;38:25–46. doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao Y., Gan L., Ren L., Lin Y., Ma C., Lin X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022;1788 doi: 10.1016/j.brainres.2022.147937. [DOI] [PubMed] [Google Scholar]
- 138.Cong Y., Baimanov D., Zhou Y., Chen C., Wang L. Penetration and translocation of functional inorganic nanomaterials into biological barriers. Adv. Drug Deliv. Rev. 2022;191 doi: 10.1016/j.addr.2022.114615. [DOI] [PubMed] [Google Scholar]
- 139.Wang C., Wu B., Wu Y., Song X., Zhang S., Liu Z. Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood-brain barrier for imaging and therapy of brain tumors. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201909369. [DOI] [Google Scholar]
- 140.Meng Y., Reilly R.M., Pezo R.C., Trudeau M., Sahgal A., Singnurkar A., Perry J., Myrehaug S., Pople C.B., Davidson B., Llinas M., Hyen C., Huang Y., Hamani C., Suppiah S., Hynynen K., Lipsman N. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abj4011. eabj4011. [DOI] [PubMed] [Google Scholar]
- 141.Curley C.T., Mead B.P., Negron K., Kim N., Garrison W.J., Miller G.W., Kingsmore K.M., Thim E.A., Song J., Munson J.M., Klibanov A.L., Suk J.S., Hanes J., Price R.J. Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay1344. eaay1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steeg P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021;18:696–714. doi: 10.1038/s41571-021-00529-6. [DOI] [PubMed] [Google Scholar]
- 143.Xu L., Nirwane A., Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2019;4:78–82. doi: 10.1136/svn-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pozzi A., Yurchenco P.D., Iozzo R.V. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jayadev R., Sherwood D.R. Basement membranes. Curr. Biol. 2017;27:R207–R211. doi: 10.1016/j.cub.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 146.Mantaj J., Abu Shams T., Enlo Scott Z., Swedrowska M., Vllasaliu D. Role of the basement membrane as an intestinal barrier to absorption of macromolecules and nanoparticles. Mol. Pharm. 2018;15:5802–5808. doi: 10.1021/acs.molpharmaceut.8b01053. [DOI] [PubMed] [Google Scholar]
- 147.Wang Q., Liang Q., Dou J., Zhou H., Zeng C., Pan H., Shen Y., Li Q., Liu Y., Leong D.T., Jiang W., Wang Y. Breaking through the basement membrane barrier to improve nanotherapeutic delivery to tumours. Nat. Nanotechnol. 2023 doi: 10.1038/s41565-023-01498-w. [DOI] [PubMed] [Google Scholar]
- 148.Bahr J.C., Li X.Y., Feinberg T.Y., Jiang L., Weiss S.J. Divergent regulation of basement membrane trafficking by human macrophages and cancer cells. Nat. Commun. 2022;13:6409. doi: 10.1038/s41467-022-34087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gimbrone M.A., García Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu Q., Chen G., Chen G., Wu H., Yang Y., Mai Z., Sun R., Luan P., Guo C., Yu M., Peng Z., Yu Z. NO-dependent vasodilation and deep tumor penetration for cascade-amplified antitumor performance. J. Control. Release. 2022;347:389–399. doi: 10.1016/j.jconrel.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 151.Weidinger A., Kozlov A. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feng Q., Li Y., Wang N., Hao Y., Chang J., Wang Z., Zhang X., Zhang Z., Wang L. A Biomimetic nanogenerator of reactive nitrogen species based on battlefield transfer strategy for enhanced immunotherapy. Small. 2020;16 doi: 10.1002/smll.202002138. [DOI] [PubMed] [Google Scholar]
- 153.Hu C., Cun X., Ruan S., Liu R., Xiao W., Yang X., Yang Y., Yang C., Gao H. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. doi: 10.1016/j.biomaterials.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 154.Miller M.A., Chandra R., Cuccarese M.F., Pfirschke C., Engblom C., Stapleton S., Adhikary U., Kohler R.H., Mohan J.F., Pittet M.J., Weissleder R. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal0225. eaal0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hou Y., Bu W., Ai H., Lu Z., Lammers T. Stimuli-responsive nanotheranostics. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202100243. [DOI] [PubMed] [Google Scholar]
- 156.Chacko R.T., Ventura J., Zhuang J., Thayumanavan S. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012;64:836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nabid M.R., Tabatabaei Rezaei S.J., Sedghi R., Niknejad H., Entezami A.A., Oskooie H.A., Heravi M.M. Self-assembled micelles of well-defined pentaerythritol-centered amphiphilic A4B8 star-block copolymers based on PCL and PEG for hydrophobic drug delivery. Polymer. 2011;52:2799–2809. doi: 10.1016/j.polymer.2011.04.054. [DOI] [Google Scholar]
- 158.Zheng X., Pan D., Chen M., Dai X., Cai H., Zhang H., Gong Q., Gu Z., Luo K. Tunable hydrophile-lipophile balance for manipulating structural stability and tumor retention of amphiphilic nanoparticles. Adv. Mater. 2019;31 doi: 10.1002/adma.201901586. [DOI] [PubMed] [Google Scholar]
- 159.Fang J., Islam W., Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020;157:142–160. doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 160.Ikeda Imafuku M., Wang L.L.W., Rodrigues D., Shaha S., Zhao Z., Mitragotri S. Strategies to improve the EPR effect: a mechanistic perspective and clinical translation. J. Control. Release. 2022;345:512–536. doi: 10.1016/j.jconrel.2022.03.043. [DOI] [PubMed] [Google Scholar]
- 161.Perrault S.D., Walkey C., Jennings T., Fischer H.C., Chan W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 162.Li H.J., Du J.Z., Liu J., Du X.J., Shen S., Zhu Y.H., Wang X., Ye X., Nie S., Wang J. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano. 2016;10:6753–6761. doi: 10.1021/acsnano.6b02326. [DOI] [PubMed] [Google Scholar]
- 163.Hou X., Zhong D., Li Y., Mao H., Yang J., Zhang H., Luo K., Gong Q., Gu Z. Facile fabrication of multi-pocket nanoparticles with stepwise size transition for promoting deep penetration and tumor targeting. J. Nanobiotechnol. 2021;19:111. doi: 10.1186/s12951-021-00854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma M., Chen Y., Zhao M., Sui J., Guo Z., Yang Y., Xu Z., Sun Y., Fan Y., Zhang X. Hierarchical responsive micelle facilitates intratumoral penetration by acid-activated positive charge surface and size contraction. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120741. [DOI] [PubMed] [Google Scholar]
- 165.Zhao X., Yang X., Wang X., Zhao X., Zhang Y., Liu S., Anderson G.J., Kim S., Li Y., Nie G. Penetration cascade of size switchable nanosystem in desmoplastic stroma for improved pancreatic cancer therapy. ACS Nano. 2021;15:14149–14161. doi: 10.1021/acsnano.0c08860. [DOI] [PubMed] [Google Scholar]
- 166.Niu Y., Zhu J., Li Y., Shi H., Gong Y., Li R., Huo Q., Ma T., Liu Y. Size shrinkable drug delivery nanosystems and priming the tumor microenvironment for deep intratumoral penetration of nanoparticles. J. Control. Release. 2018;277:35–47. doi: 10.1016/j.jconrel.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 167.Wang J., Mao W., Lock L.L., Tang J., Sui M., Sun W., Cui H., Xu D., Shen Y. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano. 2015;9:7195–7206. doi: 10.1021/acsnano.5b02017. [DOI] [PubMed] [Google Scholar]
- 168.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M., Terada Y., Kano M.R., Miyazono K., Uesaka M., Nishiyama N., Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 169.Chen T., Tam N., Mao Y., Sun C., Wang Z., Hou Y., Xia W., Yu J., Wu L. A multi-hit therapeutic nanoplatform for hepatocellular carcinoma: dual stimuli-responsive drug release, dual-modal imaging, and in situ oxygen supply to enhance synergistic therapy. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen X., Liu L., Jiang C. Charge-reversal nanoparticles: novel targeted drug delivery carriers. Acta Pharm. Sin. B. 2016;6:261–267. doi: 10.1016/j.apsb.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He S., Li J., Cheng P., Zeng Z., Zhang C., Duan H., Pu K. Charge-reversal polymer nano-modulators for photodynamic immunotherapy of cancer. Angew. Chem. Int. Ed. 2021;60:19355–19363. doi: 10.1002/anie.202106392. [DOI] [PubMed] [Google Scholar]
- 172.Xin C., Zhang Y., Bao M., Yu C., Hou K., Wang Z. Novel carrier-free, charge-reversal and DNA-affinity nanodrugs for synergistic cascade cancer chemo-chemodynamic therapy. J. Colloid Interface Sci. 2022;606:1488–1508. doi: 10.1016/j.jcis.2021.08.121. [DOI] [PubMed] [Google Scholar]
- 173.Zhang W., Wen Y., He D.X., Wang Y.F., Liu X.L., Li C., Liang X.J. Near-infrared AIEgens as transformers to enhance tumor treatment efficacy with controllable self-assembled redox-responsive carrier-free nanodrug. Biomaterials. 2019;193:12–21. doi: 10.1016/j.biomaterials.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 174.Yin S., Gao Y., Zhang Y., Xu J., Zhu J., Zhou F., Gu X., Wang G., Li J. Reduction/oxidation-responsive hierarchical nanoparticles with self-driven degradability for enhanced tumor penetration and precise chemotherapy. ACS Appl. Mater. Interfaces. 2020;12:18273–18291. doi: 10.1021/acsami.0c00355. [DOI] [PubMed] [Google Scholar]
- 175.Li H., Ma M., Zhang J., Hou W., Chen H., Zeng D., Wang Z. Ultrasound-enhanced delivery of doxorubicin-loaded nanodiamonds from pullulan-all-trans-retinal nanoparticles for effective cancer therapy. ACS Appl. Mater. Interfaces. 2019;11:20341–20349. doi: 10.1021/acsami.9b03559. [DOI] [PubMed] [Google Scholar]
- 176.Zhang S., Wu Y., He B., Luo K., Gu Z. Biodegradable polymeric nanoparticles based on amphiphilic principle: construction and application in drug delivery. Sci. China Chem. 2014;57:461–475. doi: 10.1007/s11426-014-5076-0. [DOI] [Google Scholar]
- 177.Chen Y., Zhang X.H., Cheng D.B., Zhang Y., Liu Y., Ji L., Guo R., Chen H., Ren X.K., Chen Z., Qiao Z.Y., Wang H. Near-infrared laser-triggered in situ dimorphic transformation of BF 2 -azadipyrromethene nanoaggregates for enhanced solid tumor penetration. ACS Nano. 2020;14:3640–3650. doi: 10.1021/acsnano.0c00118. [DOI] [PubMed] [Google Scholar]
- 178.He Y., Li Z., Cong C., Ye F., Yang J., Zhang X., Yuan Y., Ma Z., Zhang K., Lin Y., Zheng L., Liang X.J., Gao D. Pyroelectric catalysis-based “nano-lymphatic” reduces tumor interstitial pressure for enhanced penetration and hydrodynamic therapy. ACS Nano. 2021;15:10488–10501. doi: 10.1021/acsnano.1c03048. [DOI] [PubMed] [Google Scholar]
- 179.Yu H., Li Y., Ling D. pH and near-infrared light-responsive micelles with hyperthermia-triggered tumor penetration reverse multidrug resistance in breast cancer. Nanomedicine (N. Y., NY, U. S.) 2016;12:449–575. doi: 10.1016/j.nano.2015.12.041. [DOI] [Google Scholar]
- 180.Pan Y., Xu C., Deng H., You Q., Zhao C., Li Y., Gao Q., Akakuru O.U., Li J., Zhang J., Wu A., Chen X. Localized NIR-II laser mediated chemodynamic therapy of glioblastoma. Nano Today. 2022;43 doi: 10.1016/j.nantod.2022.101435. [DOI] [Google Scholar]
- 181.Cao W., He Y., Zhu R., He Y., Hao Z., Zhao Q., He H., Wang S., Li C., Gao D. NIR light triggered size variable “remote-controlled cluster bomb” for deep penetration and tumor therapy. Chem. Eng. J. 2019;375 doi: 10.1016/j.cej.2019.122080. [DOI] [Google Scholar]
- 182.Jin H., Zhu T., Huang X., Sun M., Li H., Zhu X., Liu M., Xie Y., Huang W., Yan D. ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: light-triggered size-reducing and enhanced tumor penetration. Biomaterials. 2019;211:68–80. doi: 10.1016/j.biomaterials.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 183.Wang K., Tu Y., Yao W., Zong Q., Xiao X., Yang R.M., Jiang X.Q., Yuan Y. Size-switchable nanoparticles with self-destructive and tumor penetration characteristics for site-specific phototherapy of cancer. ACS Appl. Mater. Interfaces. 2020;12:6933–6943. doi: 10.1021/acsami.9b21525. [DOI] [PubMed] [Google Scholar]
- 184.He P., Lei Q., Yang B., Shang T., Shi J., Ouyang Q., Wang W., Xue L., Kong F., Li Z., Huang J., Liu L., Guo J., Brinker C.J., Liu K., Zhu W. Dual-stage irradiation of size-switchable albumin nanocluster for cascaded tumor enhanced penetration and photothermal therapy. ACS Nano. 2022;16:13919–13932. doi: 10.1021/acsnano.2c02965. [DOI] [PubMed] [Google Scholar]
- 185.Epstein Barash H., Orbey G., Polat B.E., Ewoldt R.H., Feshitan J., Langer R., Borden M.A., Kohane D.S. A microcomposite hydrogel for repeated on-demand ultrasound-triggered drug delivery. Biomaterials. 2010;31:5208–5217. doi: 10.1016/j.biomaterials.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li H.L., Feng Y., Luo Q., Li Z.Q., Li X., Gan H.T., Gu Z.W., Gong Q.Y., Luo K. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics. 2023;13:5386–5417. doi: 10.7150/thno.87854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Behrens S., Appel I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016;39:89–96. doi: 10.1016/j.copbio.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 188.Wang L., Razzaq M.Y., Rudolph T., Heuchel M., Nöchel U., Mansfeld U., Jiang Y., Gould O.E.C., Behl M., Kratz K., Lendlein A. Reprogrammable, magnetically controlled polymeric nanocomposite actuators. Mater. Horiz. 2018;5:861–867. doi: 10.1039/C8MH00266E. [DOI] [Google Scholar]
- 189.Zhu Y., Song Y., Cao Z., Dong L., Lu Y., Yang X., Wang J. Magnetically actuated active deep tumor penetration of deformable large nanocarriers for enhanced cancer therapy. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202103655. [DOI] [Google Scholar]
- 190.Daocheng W., Mingxi W. A novel fluoride anion modified gelatin nanogel system for ultrasound-triggered. drug release. 2008;4:32–45. doi: 10.18433/J3988J. [DOI] [PubMed] [Google Scholar]
- 191.Kotopoulis S., Lam C., Haugse R., Snipstad S., Murvold E., Jouleh T., Berg S., Hansen R., Popa M., Mc Cormack E., Gilja O.H., Poortinga A. Formulation and characterisation of drug-loaded antibubbles for image-guided and ultrasound-triggered drug delivery. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Batchelor D.V.B., Abou Saleh R.H., Coletta P.L., McLaughlan JamesR., Peyman S.A., Evans S.D. Nested nanobubbles for ultrasound-triggered drug release. ACS Appl. Mater. Interfaces. 2020 doi: 10.1021/acsami.0c07022. acsami. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guo H., Xu M., Cao Z., Li W., Chen L., Xie X., Wang W., Liu J. Ultrasound-assisted miR-122-loaded polymeric nanodroplets for hepatocellular carcinoma gene therapy. Mol. Pharm. 2020;17:541–553. doi: 10.1021/acs.molpharmaceut.9b00983. [DOI] [PubMed] [Google Scholar]
- 194.Luo Q., Duan Z.Y., Li X.L., Gu L., Ren L., Zhu H.Y., Tian X.H., Chen R.J., Zhang H., Gong Q.Y., Gu Z.W., Luo K. Branched polymer-based redox/enzyme-activatable photodynamic nanoagent to trigger STING-dependent immune responses for enhanced therapeutic effect. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202110408. [DOI] [Google Scholar]
- 195.Tan P., Cai H., Wei Q., Tang X., Zhang Q., Kopytynski M., Yang J., Yi Y., Zhang H., Gong Q., Gu Z., Chen R., Luo K. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121061. [DOI] [PubMed] [Google Scholar]
- 196.Liu M., Huang L., Zhang W., Wang X., Geng Y., Zhang Y., Wang L., Zhang W., Zhang Y.-J., Xiao S., Bao Y., Xiong M., Wang J. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat. Nanotechnol. 2022;17:541–551. doi: 10.1038/s41565-022-01085-5. [DOI] [PubMed] [Google Scholar]
- 197.Ou Y., Chen K., Cai H., Zhang H., Gong Q., Wang J., Chen W., Luo K. Enzyme/pH-sensitive polyHPMA-DOX conjugate as a biocompatible and efficient anticancer agent. Biomater. Sci. 2018;6:1177–1188. doi: 10.1039/C8BM00095F. [DOI] [PubMed] [Google Scholar]
- 198.Feng Y., Long Y., Guo J., Yang X., Song H. Redox- and pH-responsive water-soluble flexible organic frameworks realize synergistic tumor photodynamic and chemotherapeutic therapy. Macromol. Rapid Commun. 2023;44 doi: 10.1002/marc.202200690. [DOI] [PubMed] [Google Scholar]
- 199.Guo M., Zhang X., Liu J., Gao F., Zhang X., Hu X., Li B., Zhang X., Zhou H., Bai R., Wang Y., Li J., Liu Y., Gu Z., Chen C. Few-layer bismuthene for checkpoint knockdown enhanced cancer immunotherapy with rapid clearance and sequentially triggered one-for-all strategy. ACS Nano. 2020;14:15700–15713. doi: 10.1021/acsnano.0c06656. [DOI] [PubMed] [Google Scholar]
- 200.Li J.H., Zhang X.Q., Zhao M.Y., Wu L.H., Luo K., Pu Y.J., He B. Tumor-pH-Sensitive PLLA-based microsphere with acid cleavable acetal bonds on the backbone for efficient localized chemotherapy. Biomacromolecules. 2018;19:3140–3148. doi: 10.1021/acs.biomac.8b00734. [DOI] [PubMed] [Google Scholar]
- 201.Li X., Yu L., Zhang C., Niu X., Sun M., Yan Z., Wang W., Yuan Z. Tumor acid microenvironment-activated self-targeting & splitting gold nanoassembly for tumor chemo-radiotherapy. Bioact. Mater. 2022;7:377–388. doi: 10.1016/j.bioactmat.2021.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xie X., Feng Y., Zhang H., Su Q., Song T., Yang G., Li N., Wei X., Li T., Qin X., Li S., Wu C., Zhang X., Wang G., Liu Y., Yang H. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022;16:107–119. doi: 10.1016/j.bioactmat.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li J., Wang Y., Xu C., Yu Q., Wang X., Xie H., Tian L., Qiu Y., Guo R., Lu Z., Li M., He Q. Rapid pH-responsive self-disintegrating nanoassemblies balance tumor accumulation and penetration for enhanced anti-breast cancer therapy. Acta Biomater. 2021;134:546–558. doi: 10.1016/j.actbio.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 204.Li H.J., Du J.Z., Du X.J., Xu C.F., Sun C.Y., Wang H.X., Cao Z.T., Yang X.Z., Zhu Y.H., Nie S., Wang J. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. USA. 2016;113:4164–4169. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gu L., Duan Z., Chen X., Li X., Luo Q., Bhamra A., Pan D., Zhu H., Tian X., Chen R., Gu Z., Zhang H., Qian Z., Gong Q., Luo K. A transformable amphiphilic and block polymer-dendron conjugate for enhanced tumor penetration and retention with cellular homeostasis perturbation via membrane flow. Adv. Mater. 2022;34 doi: 10.1002/adma.202200048. [DOI] [PubMed] [Google Scholar]
- 206.Nakase I., Takeuchi T., Tanaka G., Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv. Drug Deliv. Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 207.Said Hassane F., Saleh A.F., Abes R., Gait M.J., Lebleu B. Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell. Mol. Life Sci. 2010;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Duchardt F., Fotin Mleczek M., Schwarz H., Fischer R., Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 209.Jones A.T. Gateways and tools for drug delivery: endocytic pathways and the cellular dynamics of cell penetrating peptides. Int. J. Pharm. 2008;354:34–38. doi: 10.1016/j.ijpharm.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 210.Zhu Z., Wang Q., Chen X., Wang Q., Yan C., Zhao X., Zhao W., Zhu W. An enzyme-activatable aggregation-induced-emission probe: intraoperative pathological fluorescent diagnosis of pancreatic cancer via specific cathepsin E. Adv. Mater. 2022;34 doi: 10.1002/adma.202107444. [DOI] [PubMed] [Google Scholar]
- 211.Govindarajan S., Sivakumar J., Garimidi P., Rangaraj N., Kumar J.M., Rao N.M., Gopal V. Targeting human epidermal growth factor receptor 2 by a cell-penetrating peptide-affibody bioconjugate. Biomaterials. 2012;33:2570–2582. doi: 10.1016/j.biomaterials.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 212.He X., Chen X., Liu L., Zhang Y., Lu Y., Zhang Y., Chen Q., Ruan C., Guo Q., Li C., Sun T., Jiang C. Sequentially triggered nanoparticles with tumor penetration and intelligent drug release for pancreatic cancer therapy. Adv. Sci. 2018;5 doi: 10.1002/advs.201701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jiang T., Wang T., Li T., Ma Y., Shen S., He B., Mo R. Enhanced transdermal drug delivery by transfersome-embedded oligopeptide hydrogel for topical chemotherapy of melanoma. ACS Nano. 2018;12:9693–9701. doi: 10.1021/acsnano.8b03800. [DOI] [PubMed] [Google Scholar]
- 214.Ding Y., Liu J., Zhang Y., Li X., Ou H., Cheng T., Ma L., An Y., Liu J., Huang F., Liu Y., Shi L. A novel strategy based on a ligand-switchable nanoparticle delivery system for deep tumor penetration. Nanoscale Horiz. 2019;4:658–666. doi: 10.1039/c8nh00415c. [DOI] [Google Scholar]
- 215.Nakase I., Konishi Y., Ueda M., Saji H., Futaki S. Accumulation of arginine-rich cell-penetrating peptides in tumors and the potential for anticancer drug delivery in vivo. J. Control. Release. 2012;159:181–188. doi: 10.1016/j.jconrel.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 216.Nakase I., Akita H., Kogure K., Gräslund A., Langel Ü., Harashima H., Futaki S. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc. Chem. Res. 2012;45:1132–1139. doi: 10.1021/ar200256e. [DOI] [PubMed] [Google Scholar]
- 217.Wei Y., Zhang M., Jiao P., Zhang X., Yang G., Xu X. Intracellular paclitaxel delivery facilitated by a dual-functional CPP with a hydrophobic hairpin tail. ACS Appl. Mater. Interfaces. 2021;13:4853–4860. doi: 10.1021/acsami.0c20180. [DOI] [PubMed] [Google Scholar]
- 218.Liu Y., Mei L., Xu C., Yu Q., Shi K., Zhang L., Wang Y., Zhang Q., Gao H., Zhang Z., He Q. Dual receptor recognizing cell penetrating peptide for selective targeting, efficient intratumoral diffusion and synthesized anti-glioma therapy. Theranostics. 2016;6:177–191. doi: 10.7150/thno.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tang J., Zhang L., Fu H., Kuang Q., Gao H., Zhang Z., He Q. A detachable coating of cholesterol-anchored PEG improves tumor targeting of cell-penetrating peptide-modified liposomes. Acta Pharm. Sin. B. 2014;4:67–73. doi: 10.1016/j.apsb.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cho H.J., Park S.J., Lee Y.S., Kim S. Theranostic iRGD peptide containing cisplatin prodrug: dual-cargo tumor penetration for improved imaging and therapy. J. Control. Release. 2019;300:73–80. doi: 10.1016/j.jconrel.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 221.Hurtado De Mendoza T., Mose E.S., Botta G.P., Braun G.B., Kotamraju V.R., French R.P., Suzuki K., Miyamura N., Teesalu T., Ruoslahti E., Lowy A.M., Sugahara K.N. Tumor-penetrating therapy for β5 integrin-rich pancreas cancer. Nat. Commun. 2021;12:1541. doi: 10.1038/s41467-021-21858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Saifi M.A., Sathish G., Bazaz M.R., Godugu C. Exploration of tumor penetrating peptide iRGD as a potential strategy to enhance tumor penetration of cancer nanotherapeutics. Biochim. Biophys. Acta, Rev. Cancer. 2023;1878 doi: 10.1016/j.bbcan.2023.188895. [DOI] [PubMed] [Google Scholar]
- 223.Jiang Y., Pang X., Liu R., Xiao Q., Wang P., Leung A.W., Luan Y., Xu C. Design of an amphiphilic iRGD peptide and self-assembling nanovesicles for improving tumor accumulation and penetration and the photodynamic efficacy of the photosensitizer. ACS Appl. Mater. Interfaces. 2018;10:31674–31685. doi: 10.1021/acsami.8b11699. [DOI] [PubMed] [Google Scholar]
- 224.Ma S., Zhou J., Zhang Y., He Y., Jiang Q., Yue D., Xu X., Gu Z. Highly stable fluorinated nanocarriers with irgd for overcoming the stability dilemma and enhancing tumor penetration in an orthotopic breast cancer. ACS Appl. Mater. Interfaces. 2016;8:28468–28479. doi: 10.1021/acsami.6b09633. [DOI] [PubMed] [Google Scholar]
- 225.Ding N., Zou Z., Sha H., Su S., Qian H., Meng F., Chen F., Du S., Zhou S., Chen H., Zhang L., Yang J., Wei J., Liu B. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat. Commun. 2019;10:1336. doi: 10.1038/s41467-019-09296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cordero Cervantes D., Zurzolo C. Peering into tunneling nanotubes—the path forward. EMBO J. 2021;40 doi: 10.15252/embj.2020105789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pinto G., Brou C., Zurzolo C. Tunneling nanotubes: the fuel of tumor progression? Trends Cancer. 2020;6:874–888. doi: 10.1016/j.trecan.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 228.Maugeri M., Nawaz M., Papadimitriou A., Angerfors A., Camponeschi A., Na M., Hölttä M., Skantze P., Johansson S., Sundqvist M., Lindquist J., Kjellman T., Mårtensson I.L., Jin T., Sunnerhagen P., Östman S., Lindfors L., Valadi H. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019;10:4333. doi: 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lenzini S., Bargi R., Chung G., Shin J.W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 2020;15:217–223. doi: 10.1038/s41565-020-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Guo S., Wang Y., Miao L., Xu Z., Lin C.M., Zhang Y., Huang L. Lipid-coated cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano. 2013;7:9896–9904. doi: 10.1021/nn403606m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhao D., Tao W., Li S., Chen Y., Sun Y., He Z., Sun B., Sun J. Apoptotic body-mediated intercellular delivery for enhanced drug penetration and whole tumor destruction. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg0880. eabg0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ju C., Mo R., Xue J., Zhang L., Zhao Z., Xue L., Ping Q., Zhang C. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angew. Chem. Int. Ed. 2014;53:6253–6258. doi: 10.1002/anie.201311227. [DOI] [PubMed] [Google Scholar]
- 233.Wang C., Chen S., Wang Y., Liu X., Hu F., Sun J., Yuan H. Lipase-triggered water-responsive “pandora's box” for cancer therapy: toward induced neighboring effect and enhanced drug penetration. Adv. Mater. 2018;30 doi: 10.1002/adma.201706407. [DOI] [PubMed] [Google Scholar]
- 234.Luo L., Qi Y., Zhong H., Jiang S., Zhang H., Cai H., Wu Y., Gu Z., Gong Q., Luo K. GSH-sensitive polymeric prodrug: synthesis and loading with photosensitizers as nanoscale chemo-photodynamic anti-cancer nanomedicine. Acta Pharm. Sin. B. 2022;12:424–436. doi: 10.1016/j.apsb.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Duan Z., Luo Q., Dai X., Li X., Gu L., Zhu H., Tian X., Zhang H., Gong Q., Gu Z., Luo K. Synergistic therapy of a naturally inspired glycopolymer-based biomimetic nanomedicine harnessing tumor genomic instability. Adv. Mater. 2021;33 doi: 10.1002/adma.202104594. [DOI] [PubMed] [Google Scholar]
- 236.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J., MacMillan P., Zhang Y., Rajesh N.U., Hoang T., Wu J.L.Y., Wilhelm S., Zilman A., Gadde S., Sulaiman A., Ouyang B., Lin Z., Wang L., Egeblad M., Chan W.C.W. The entry of nanoparticles into solid tumours. Nat. Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 237.Nel A., Ruoslahti E., Meng H. New insights into “permeability” as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano. 2017;11:9567–9569. doi: 10.1021/acsnano.7b07214. [DOI] [PubMed] [Google Scholar]
- 238.Zhu X., Jin K., Huang Y., Pang Z. Brain drug delivery by adsorption-mediated transcytosis, Brain Target. Drug Deliv. Syst. 2019:159–183. doi: 10.1016/B978-0-12-814001-7.00007-X. [DOI] [Google Scholar]
- 239.Chen H., Song H., Luo Y., Li C., Wang Y., Liu J., Luo F., Fan H., Li X., Sun T., Jiang C. Transcytosis mediated deep tumor penetration for enhanced chemotherapy and immune activation of pancreatic cancer. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202214937. [DOI] [Google Scholar]
- 240.Miura S., Suzuki H., Bae Y.H. A multilayered cell culture model for transport study in solid tumors: evaluation of tissue penetration of polyethyleneimine based cationic micelles. Nano Today. 2014;9:695–704. doi: 10.1016/j.nantod.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen S., Zhong Y., Fan W., Xiang J., Wang G., Zhou Q., Wang J., Geng Y., Sun R., Zhang Z., Piao Y., Wang J., Zhuo J., Cong H., Jiang H., Ling J., Li Z., Yang D., Yao X., Xu X., Zhou Z., Tang J., Shen Y. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 2021;5:1019–1037. doi: 10.1038/s41551-021-00701-4. [DOI] [PubMed] [Google Scholar]
- 242.Syvänen S., Edén D., Sehlin D. Cationization increases brain distribution of an amyloid-beta protofibril selective F(ab’)2 fragment. Biochem. Biophys. Res. Commun. 2017;493:120–125. doi: 10.1016/j.bbrc.2017.09.065. [DOI] [PubMed] [Google Scholar]
- 243.Benjaminsen R.V., Mattebjerg M.A., Henriksen J.R., Moghimi S.M., Andresen T.L. The possible “proton sponge ” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013;21:149–157. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhou Q., Shao S., Wang J., Xu C., Xiang J., Piao Y., Zhou Z., Yu Q., Tang J., Liu X., Gan Z., Mo R., Gu Z., Shen Y. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 245.Yan H., Xu P., Ma H., Li Y., Zhang R., Cong H., Yu B., Shen Y. Enzyme-triggered transcytosis of drug carrier system for deep penetration into hepatoma tumors. Biomaterials. 2023;301 doi: 10.1016/j.biomaterials.2023.122213. [DOI] [PubMed] [Google Scholar]
- 246.Zhang L., Wang D., Yang K., Sheng D., Tan B., Wang Z., Ran H., Yi H., Zhong Y., Lin H., Chen Y. Mitochondria-targeted artificial “nano-RBCs” for amplified synergistic cancer phototherapy by a single NIR irradiation. Adv. Sci. 2018;5 doi: 10.1002/advs.201800049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu C.X., Wang B., Zhu W.P., Xu Y.F., Yang Y.Y., Qian X.H. An endoplasmic reticulum (ER)-targeting DNA nanodevice for autophagy-dependent degradation of proteins in membrane-bound organelles. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202205509. [DOI] [PubMed] [Google Scholar]
- 248.Ramírez García P.D., Retamal J.S., Shenoy P., Imlach W., Sykes M., Truong N., Constandil L., Pelissier T., Nowell C.J., Khor S.Y., Layani L.M., Lumb C., Poole D.P., Lieu T., Stewart G.D., Mai Q.N., Jensen D.D., Latorre R., Scheff N.N., Schmidt B.L., Quinn J.F., Whittaker M.R., Veldhuis N.A., Davis T.P., Bunnett N.W. A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol. 2019;14:1150–1159. doi: 10.1038/s41565-019-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Towers C.G., Thorburn A. Targeting the lysosome for cancer therapy. Cancer Discov. 2017;7:1218–1220. doi: 10.1158/2159-8290.CD-17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bonam S.R., Wang F., Muller S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019;18:923–948. doi: 10.1038/s41573-019-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Borkowska M., Siek M., Kolygina D.V., Sobolev Y.I., Lach S., Kumar S., Cho Y.K., Kandere Grzybowska K., Grzybowski B.A. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol. 2020;15:331–341. doi: 10.1038/s41565-020-0643-3. [DOI] [PubMed] [Google Scholar]
- 252.Wang Y., Niu G., Zhai S., Zhang W., Xing D. Specific photoacoustic cavitation through nucleus targeted nanoparticles for high-efficiency tumor therapy. Nano Res. 2020;13:719–728. doi: 10.1007/s12274-020-2681-4. [DOI] [Google Scholar]
- 253.Wu H., Carvalho P., Voeltz G.K. Here, there, and everywhere: the importance of ER membrane contact sites. Science. 2018;361 doi: 10.1126/science.aan5835. eaan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Scorrano L., De Matteis M.A., Emr S., Giordano F., Hajnóczky G., Kornmann B., Lackner L.L., Levine T.P., Pellegrini L., Reinisch K., Rizzuto R., Simmen T., Stenmark H., Ungermann C., Schuldiner M. Coming together to define membrane contact sites. Nat. Commun. 2019;10:1287. doi: 10.1038/s41467-019-09253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Elbaz Alon Y., Guo Y., Segev N., Harel M., Quinnell D.E., Geiger T., Avinoam O., Li D., Nunnari J. PDZD8 interacts with Protrudin and Rab7 at ER-late endosome membrane contact sites associated with mitochondria. Nat. Commun. 2020;11:3645. doi: 10.1038/s41467-020-17451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Muallem S., Chung W.Y., Jha A., Ahuja M. Lipids at membrane contact sites: cell signaling and ion transport. EMBO Rep. 2017;18:1893–1904. doi: 10.15252/embr.201744331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Raiborg C., Wenzel E.M., Pedersen N.M., Olsvik H., Schink K.O., Schultz S.W., Vietri M., Nisi V., Bucci C., Brech A., Johansen T., Stenmark H. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- 258.King C., Sengupta P., Seo A.Y., Lippincott Schwartz J. ER membranes exhibit phase behavior at sites of organelle contact. Proc. Natl. Acad. Sci. USA. 2020;117:7225–7235. doi: 10.1073/pnas.1910854117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Caldieri G., Barbieri E., Nappo G., Raimondi A., Bonora M., Conte A., Verhoef L.G.G.C., Confalonieri S., Malabarba M.G., Bianchi F., Cuomo A., Bonaldi T., Martini E., Mazza D., Pinton P., Tacchetti C., Polo S., Di Fiore P.P., Sigismund S. Reticulon 3-dependent ER-PM contact sites control EGFR nonclathrin endocytosis. Science. 2017;356:617–624. doi: 10.1126/science.aah6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wenzel E.M., Elfmark L.A., Stenmark H., Raiborg C. ER as master regulator of membrane trafficking and organelle function. J. Cell Biol. 2022;221 doi: 10.1083/jcb.202205135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wu H., Voeltz G.K. Reticulon-3 promotes endosome maturation at ER membrane contact sites. Dev. Cell. 2021;56:52–66. doi: 10.1016/j.devcel.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang H. Lipid transfer at ER-isolation membrane contacts. Nat. Rev. Mol. Cell Biol. 2020;21 doi: 10.1038/s41580-020-0212-5. 121-121. [DOI] [PubMed] [Google Scholar]
- 263.Malek M., Wawrzyniak A.M., Koch P., Lüchtenborg C., Hessenberger M., Sachsenheimer T., Jang W., Brügger B., Haucke V. Inositol triphosphate-triggered calcium release blocks lipid exchange at endoplasmic reticulum-Golgi contact sites. Nat. Commun. 2021;12:2673. doi: 10.1038/s41467-021-22882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pedersen N.M., Wenzel E.M., Wang L., Antoine S., Chavrier P., Stenmark H., Raiborg C. Protrudin-mediated ER-endosome contact sites promote MT1-MMP exocytosis and cell invasion. J. Cell Biol. 2020;219 doi: 10.1083/jcb.202003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gallo A., Vannier C., Galli T. Endoplasmic Reticulum-plasma membrane associations: structures and functions. Annu. Rev. Cell Dev. Biol. 2016;32:279–301. doi: 10.1146/annurev-cellbio-111315-125024. [DOI] [PubMed] [Google Scholar]
- 266.Vance J.E. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 267.Wijdeven R.H., Jongsma M.L.M., Neefjes J., Berlin I. ER contact sites direct late endosome transport. Bioessays. 2015;37:1298–1302. doi: 10.1002/bies.201500095. [DOI] [PubMed] [Google Scholar]
- 268.Kang Y., Liu J., Jiang Y., Yin S., Huang Z., Zhang Y., Wu J., Chen L., Shao L. Understanding the interactions between inorganic-based nanomaterials and biological membranes. Adv. Drug Deliv. Rev. 2021;175 doi: 10.1016/j.addr.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 269.Jang H.L., Sengupta S. Transcellular transfer of nanomedicine. Nat. Nanotechnol. 2019;14:731–732. doi: 10.1038/s41565-019-0494-y. [DOI] [PubMed] [Google Scholar]
- 270.Gu L., Duan Z.Y., Li X., Li X., Li Y.G., Li X.L., Xu G., Gao P., Zhang H., Gu Z.W., Chen J., Gong Q.Y., Luo K. Enzyme-triggered deep tumor penetration of a dual-drug nanomedicine enables an enhanced cancer combination therapy. Bioact. Mater. 2023;26:102–115. doi: 10.1016/j.bioactmat.2023.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zeng Y., Li S., Zhang S., Wang L., Yuan H., Hu F. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B. 2022;12:3233–3254. doi: 10.1016/j.apsb.2022.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Fang R.H., Gao W., Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023;20:33–48. doi: 10.1038/s41571-022-00699-x. [DOI] [PubMed] [Google Scholar]
- 273.Tang Y., Wang X., Li J., Nie Y., Liao G., Yu Y., Li C. Overcoming the reticuloendothelial system barrier to drug delivery with a “Don’t-Eat-Us” strategy. ACS Nano. 2019;13:13015–13026. doi: 10.1021/acsnano.9b05679. [DOI] [PubMed] [Google Scholar]
- 274.Zhu R., Jiang W., Pu Y.J., Luo K., Wu Y., He B., Gu Z.W. Functionalization of magnetic nanoparticles with peptide dendrimers. J. Mater. Chem. 2011;21:5464–5474. doi: 10.1039/C0JM02752A. [DOI] [Google Scholar]
- 275.Luo K., Liu G., Zhang X., She W., He B., Nie Y., Li L., Wu Y., Zhang Z., Gong Q., Gao F., Song B., Ai H., Gu Z. Functional L-lysine dendritic macromolecules as liver-imaging probes. Macromol. Biosci. 2009;9:1227–1236. doi: 10.1002/mabi.200900231. [DOI] [PubMed] [Google Scholar]
- 276.Zhang W., Jiang Y., He Y., Boucetta H., Wu J., Chen Z., He W. Lipid carriers for mRNA delivery. Acta Pharm. Sin. B. 2022 doi: 10.1016/j.apsb.2022.11.026. S2211383522004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liu J., Ma C., Shi S., Liu H., Wen W., Zhang X., Wu Z., Wang S. A general controllable release amplification strategy of liposomes for single-particle collision electrochemical biosensing. Biosens. Bioelectron. 2022;207 doi: 10.1016/j.bios.2022.114182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Fanciullino R., Ciccolini J., Milano G. COVID-19 vaccine race: watch your step for cancer patients. Br. J. Cancer. 2021;124:860–861. doi: 10.1038/s41416-020-01219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huang H., Zhang C., Yang S., Xiao W., Zheng Q., Song X. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J. Control. Release. 2021;335:449–456. doi: 10.1016/j.jconrel.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J., MacMillan P., Zhang Y., Rajesh N.U., Hoang T., Wu J.L.Y., Wilhelm S., Zilman A., Gadde S., Sulaiman A., Ouyang B., Lin Z., Wang L., Egeblad M., Chan W.C.W. The entry of nanoparticles into solid tumours. Nat. Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 281.Zhou F., Feng B., Wang T., Wang D., Meng Q., Zeng J., Zhang Z., Wang S., Yu H., Li Y. Programmed multiresponsive vesicles for enhanced tumor penetration and combination therapy of triple-negative breast cancer. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201606530. [DOI] [Google Scholar]
- 282.Giulimondi F., Vulpis E., Digiacomo L., Giuli M.V., Mancusi A., Capriotti A.L., Laganà A., Cerrato A., Zenezini Chiozzi R., Nicoletti C., Amenitsch H., Cardarelli F., Masuelli L., Bei R., Screpanti I., Pozzi D., Zingoni A., Checquolo S., Caracciolo G. Opsonin-deficient nucleoproteic corona endows unPEGylated liposomes with stealth properties in vivo. ACS Nano. 2022;16:2088–2100. doi: 10.1021/acsnano.1c07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kristensen K., Engel T.B., Stensballe A., Simonsen J.B., Andresen T.L. The hard protein corona of stealth liposomes is sparse. J. Control. Release. 2019;307:1–15. doi: 10.1016/j.jconrel.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 284.Popilski H., Feinshtein V., Kleiman S., Mattarei A., Garofalo M., Salmaso S., Stepensky D. Doxorubicin liposomes cell penetration enhancement and its potential drawbacks for the tumor targeting efficiency. Int. J. Pharm. 2021;592 doi: 10.1016/j.ijpharm.2020.120012. [DOI] [PubMed] [Google Scholar]
- 285.Tahara Y., Yoshikawa T., Sato H., Mori Y., Zahangir M.H., Kishimura A., Mori T., Katayama Y. Encapsulation of a nitric oxide donor into a liposome to boost the enhanced permeation and retention (EPR) effect. MedChemComm. 2017;8:415–421. doi: 10.1039/C6MD00614K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chen H., Fan X., Zhao Y., Zhi D., Cui S., Zhang E., Lan H., Du J., Zhang Z., Zhang S., Zhen Y. Stimuli-responsive polysaccharide enveloped liposome for targeting and penetrating delivery of survivin-shRNA into breast tumor. ACS Appl. Mater. Interfaces. 2020;12:22074–22087. doi: 10.1021/acsami.9b22440. [DOI] [PubMed] [Google Scholar]
- 287.Thurston G., McLean J.W., Rizen M., Baluk P., Haskell A., Murphy T.J., Hanahan D., McDonald D.M. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J. Clin. Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang H.X., Zuo Z.Q., Du J.Z., Wang Y.C., Sun R., Cao Z.T., Ye X.D., Wang J.L., Leong K.W., Wang J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today. 2016;11:133–144. doi: 10.1016/j.nantod.2016.04.008. [DOI] [Google Scholar]
- 289.Widjaya A.S. Tumor-permeable smart liposomes by modulating the tumor microenvironment to improve the chemotherapy. J. Control. Release. 2022;344:62–79. doi: 10.1016/j.jconrel.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 290.Pan S., Pei L., Zhang A., Zhang Y., Zhang C., Huang M., Huang Z., Liu B., Wang L., Ma L., Zhang Q., Cui D. Passion fruit-like exosome-PMA/Au-BSA@Ce6 nanovehicles for real-time fluorescence imaging and enhanced targeted photodynamic therapy with deep penetration and superior retention behavior in tumor. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119606. [DOI] [PubMed] [Google Scholar]
- 291.Zhu D., Duo Y., Suo M., Zhao Y., Xia L., Zheng Z., Li Y., Tang B.Z. Tumor-exocytosed exosome/aggregation-induced emission luminogen hybrid nanovesicles facilitate efficient tumor penetration and photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:13836–13843. doi: 10.1002/anie.202003672. [DOI] [PubMed] [Google Scholar]
- 292.Yong T., Zhang X., Bie N., Zhang H., Zhang X., Li F., Hakeem A., Hu J., Gan L., Santos H.A., Yang X. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019;10:3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., Batrakova E.V. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C., Ju S., Mu J., Zhang L., Steinman L., Miller D., Zhang H.G. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yuan D., Zhao Y., Banks W.A., Bullock K.M., Haney M., Batrakova E., Kabanov A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wei Z., Zhang X., Yong T., Bie N., Zhan G., Li X., Liang Q., Li J., Yu J., Huang G., Yan Y., Zhang Z., Zhang B., Gan L., Huang B., Yang X. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 2021;12:440. doi: 10.1038/s41467-020-20723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Dad H.A., Gu T.W., Zhu A.Q., Huang L.Q., Peng L.H. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021;29:13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Xu X.H., Yuan T.J., Dad H.A., Shi M.Y., Huang Y.Y., Jiang Z.H., Peng L.H. Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21:8151–8159. doi: 10.1021/acs.nanolett.1c02530. [DOI] [PubMed] [Google Scholar]
- 299.Nie D., Dai Z., Li J., Yang Y., Xi Z., Wang J., Zhang W., Qian K., Guo S., Zhu C., Wang R., Li Y., Yu M., Zhang X., Shi X., Gan Y. Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett. 2020;20:936–946. doi: 10.1021/acs.nanolett.9b03817. [DOI] [PubMed] [Google Scholar]
- 300.Miao Y., Yang Y., Guo L., Chen M., Zhou X., Zhao Y., Nie D., Gan Y., Zhang X. Cell membrane-camouflaged nanocarriers with biomimetic deformability of erythrocytes for ultralong circulation and enhanced cancer therapy. ACS Nano. 2022;16:6527–6540. doi: 10.1021/acsnano.2c00893. [DOI] [PubMed] [Google Scholar]
- 301.Liu C., Zhang W., Li Y., Chang J., Tian F., Zhao F., Ma Y., Sun J. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett. 2019;19:7836–7844. doi: 10.1021/acs.nanolett.9b02841. [DOI] [PubMed] [Google Scholar]
- 302.Shao M., Lopes D., Lopes J., Yousefiasl S., Macário Soares A., Peixoto D., Ferreira Faria I., Veiga F., Conde J., Huang Y., Chen X., Paiva-Santos A.C., Makvandi P. Exosome membrane-coated nanosystems: exploring biomedical applications in cancer diagnosis and therapy. Matter. 2023;6:761–799. doi: 10.1016/j.matt.2023.01.012. [DOI] [Google Scholar]
- 303.Xu C.H., Ye P.J., Zhou Y.C., He D.X., Wei H., Yu C.Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi: 10.1016/j.actbio.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 304.Park J.H., Mohapatra A., Zhou J., Holay M., Krishnan N., Gao W., Fang R.H., Zhang L. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wang S., Duan Y., Zhang Q., Komarla A., Gong H., Gao W., Zhang L. Drug targeting via platelet membrane-coated nanoparticles. Small Struct. 2020;1 doi: 10.1002/sstr.202000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhang Y., Chen Y., Lo C., Zhuang J., Angsantikul P., Zhang Q., Wei X., Zhou Z., Obonyo M., Fang R.H., Gao W., Zhang L. Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew. Chem. Int. Ed. 2019;58:11404–11408. doi: 10.1002/anie.201906280. [DOI] [PubMed] [Google Scholar]
- 307.Zhu X., Wang T., Jia H., Wu F. Immunocyte-derived nanodrugs for cancer therapy. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202207181. [DOI] [Google Scholar]
- 308.Duan Y., Zhou J., Zhou Z., Zhang E., Yu Y., Krishnan N., Silva Ayala D., Fang R.H., Griffiths A., Gao W., Zhang L. Extending the in vivo residence time of macrophage membrane-coated nanoparticles through genetic modification. Small. 2023 doi: 10.1002/smll.202305551. [DOI] [PubMed] [Google Scholar]
- 309.Tang Q., Sun S., Wang P., Sun L., Wang Y., Zhang L., Xu M., Chen J., Wu R., Zhang J., Gong M., Chen Q., Liang X. Genetically engineering cell membrane-coated BTO nanoparticles for MMP2-activated piezocatalysis-immunotherapy. Adv. Mater. 2023;35 doi: 10.1002/adma.202300964. [DOI] [PubMed] [Google Scholar]
- 310.Zou D., Wu Z., Yi X., Hui Y., Yang G., Liu Y., Tengjisi, Wang H., Brooks A., Wang H., Liu X., Xu Z.P., Roberts M.S., Gao H., Zhao C.X. Nanoparticle elasticity regulates the formation of cell membrane-coated nanoparticles and their nano-bio interactions. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2214757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhou H., Fan Z., Lemons P.K., Cheng H. A facile approach to functionalize cell membrane-coated nanoparticles. Theranostics. 2016;6:1012–1022. doi: 10.7150/thno.15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chai Z., Hu X., Lu W. Cell membrane-coated nanoparticles for tumor-targeted drug delivery. Sci. China Mater. 2017;60:504–510. doi: 10.1007/s40843-016-5163-4. [DOI] [Google Scholar]
- 313.Wang D., Wang S., Zhou Z., Bai D., Zhang Q., Ai X., Gao W., Zhang L. White Blood Cell membrane-coated nanoparticles: recent development and medical applications. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202101349. [DOI] [PubMed] [Google Scholar]
- 314.Farjadian F., Ghasemi A., Gohari O., Roointan A., Karimi M., Hamblin M.R. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14:93–126. doi: 10.2217/nnm-2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Service R.F. Nanoparticle trojan horses gallop from the lab into the clinic. Science. 2010;330:314–315. doi: 10.1126/science.330.6002.314. [DOI] [PubMed] [Google Scholar]
- 316.Le P.N., Huynh C.K., Tran N.Q. Advances in thermosensitive polymer-grafted platforms for biomedical applications. Mater. Sci. Eng., C. 2018;92:1016–1030. doi: 10.1016/j.msec.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 317.Sanna V., Sechi M. Nanoparticle therapeutics for prostate cancer treatment. Nanomedicine (N. Y., NY, U. S.) 2012;8:S31–S36. doi: 10.1016/j.nano.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 318.Abdalla A.M.E., Xiao L., Miao Y., Huang L., Fadlallah G.M., Gauthier M., Ouyang C., Yang G. Nanotechnology promotes genetic and functional modifications of therapeutic T cells against cancer. Adv. Sci. 2020;7 doi: 10.1002/advs.201903164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Li J., Wu Y., Wang J., Xu X., Zhang A., Li Y., Zhang Z. Macrophage membrane-coated nano-gemcitabine promotes lymphocyte infiltration and synergizes antiPD-L1 to restore the tumoricidal function. ACS Nano. 2023;17:322–336. doi: 10.1021/acsnano.2c07861. [DOI] [PubMed] [Google Scholar]