Abstract
This study was designed to determine the efficacy of epalrestat on patients with diabetic foot infection (DFI) and its effects on serum inflammatory factors in the patients. Methods: The data of 80 patients with DFI treated in the First Affiliated Hospital of Jiangxi Medical College from May 2020 to May 2022 were analyzed retrospectively. Among them, patients who received routine comprehensive treatment were enrolled into the control group (n=37), and those who received epalrestat on the basis of routine comprehensive treatment were enrolled into the study group (n=43). The changes of serum inflammatory factors before and after treatment, granulation tissue grading and efficacy in the two groups were analyzed and compared, and the wound healing time, hospitalization time and adverse reactions (including nausea and vomiting, dizziness, headache, pruritus, etc.) of the two groups were statistically analyzed. The prognosis of the patients within 1 year after treatment was analyzed, and the independent risk factors of poor prognosis were analyzed through logistic regression. Results: Before treatment, the two groups were not significantly different in the levels of tumor necrosis factor-α (TNF-α), high sensitivity C-reactive protein (hs-CRP), and interleukin-6 (IL-6), while after treatment, the levels decreased significantly in both groups, with significantly lower levels in the study group than those in the control group. The study group had a significant lower proportion of patients with grade 0/grade 1 granulation tissue than the control group, and had a significantly higher proportion of patients with grade 2/grade 4 granulation tissue than the control group, but the proportion of patients with grade 3 granulation tissue in the two groups was not greatly different. The study group experienced notably shorter wound healing time and hospitalization time than the control group. A notably higher overall response rate was found in the study group than that in the control group. In addition, the total incidence of adverse reactions was not greatly different between the two groups. BMI, diabetes mellitus type, Wagner grading and classification of diabetic foot infection were found to be the risk factors affecting the prognosis of patients, and Wagner grading was an independent risk factor affecting the prognosis of patients. Conclusion: Epalrestat is effective in treating DFI, because it can lower the levels of serum inflammatory factors, shorten the time of wound healing and hospitalization, and promote the growth and recovery of granulation, without increasing adverse reactions. Therefore, it is worthy of clinical promotion.
Keywords: Epalrestat, diabetic foot infection, efficacy, serum inflammatory factors, granulation tissue
Introduction
As the incidence of diabetes mellitus (DM) increases, an increasing number of patients suffer from diabetic foot [1]. Diabetic foot is a series of foot diseases due to poor blood glucose control and peripheral nerve and vascular diseases, and diabetic foot infection (DFI) is a major complication [2]. DFI is a crucial culprit for the development and deterioration of diabetic foot, and diabetic foot complicated with deep infection is the most common cause of amputation in patients suffering from diabetic foot [3]. DFI is commonly caused by neuropathic ulcer, with a long treatment cycle, high cost, great difficulty, high disability and high mortality rate [4]. Patients with DFI often need amputation, which substantially compromises their quality of life [5], so, effective measures to improve the condition of patients with DFI is strongly correlated with their physical and mental health.
Antibiotics are an important means and method for DFI, but the drug resistance, double infection and side effects of bacteria enhance the difficulty of the treatment [6]. The advent of epalrestat provides a novel method for the treatment of DFI. Epalrestat is a specific aldose reductase inhibitor, which acts by blocking the polyol pathway [7]. With abilities to reduce oxidative stress and inhibit protein non-enzymatic glycosylation in individuals with DM, epalrestat is mainly adopted in the treatment of chronic complications of DM. Ramirez et al. [8] have revealed that epalrestat can be adopted as an aldose reductase inhibitor in the treatment of diabetic neuropathy with a low incidence of adverse reactions and is effective and safe for chronic diabetic complications. Alvarez-Rivera et al. [9] have also revealed a certain therapeutic effect of epalrestat on diabetic ocular complications. However, there are still few related studies on the application of epalrestat in DFI.
Accordingly, this study explored the efficacy of epalrestat on patients with DFI and its effects on serum inflammatory factors in the patients to offer reference to the follow-up treatment of DFI.
Materials and methods
Clinical data
The data of 80 patients with DFI treated in the First Affiliated Hospital of Jiangxi Medical College from May 2020 to May 2022 were analyzed retrospectively. Among them, patients who received routine comprehensive treatment were enrolled into the control group (n=37), and those who received epalrestat on the basis of routine comprehensive treatment were enrolled into the study group (n=43). This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Jiangxi Medical College.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients who met the diagnostic criteria of diabetic foot in the Chinese Guidelines for the Diagnosis and Treatment of Diabetic Foot [10]; (2) Patients with Wagner grade 2-3 [11]; (3) Patients who met the clinical classification criteria of grade 2-3 DFI in the diagnostic criteria of Guidelines for Diagnosis and Treatment of Diabetic Foot Infection (updated in 2019) formulated by the International Working Group on the Diabetic Foot (IWGDF) [12]; (4) Patients over 18 years old; (5) Patients with complete clinical data.
Exclusion criteria: (1) Patients with malignant tumor, severe hypoproteinemia, dysfunction of important organs, cardiovascular or cerebrovascular diseases, thyroid dysfunction or hematological diseases; (2) Patients with severe lesions requiring amputation; (3) Patients who were comorbid with severe diabetic complications except DFI and had unstable vital signs; (4) Patients who were allergic to the drugs adopted in this study or had an allergic history; (5) Patients during pregnancy or lactation.
Therapeutic regimen
Both groups were given commonly adopted treatment, including antibiotic treatment, wound treatment, control of diabetes, surgical intervention. Specifically, for the control group: Each patient was given insulin (Insulin injection: Jiangsu Wanbang Biopharmaceuticals; State Food and Drug Administration approval number: H10890001) to control the blood glucose levels, and was given symptomatic treatment against other basic diseases, such as hypertension and hyperlipidemia. For anti-infection treatment, empirical drugs (cefixime dispersible tablets: Guangzhou Baiyunshan Pharmaceutical Holdings Company Limited; State Food and Drug Administration approval number: H20030048; amoxicillin: Sichuan Yuanjian Pharmaceutical Co., Ltd.; State Food and Drug Administration approval number: H21023908) were adopted first. Additionally, wound secretions of the patient were collected for pathogenic examination. After acquiring the results of pathogen drug sensitivity test, sensitive antibacterial drugs were selected for anti-infection treatment. The infected wound was thoroughly debrided 3 days after admission, and the wound was closed by negative pressure suction foam dressing. The constant negative pressure suction was carried out at -10.6~-8.0 kPa until the wound reached the condition of skin graft repair.
For the study group: In addition to treatment in the control group, each patient in the study group received oral epalrestat tablets (Shandong DYNE Marine Biopharmaceutical Co., Ltd., State Food and Drug Administration approval number: H20050893; specification: 50 mg × 12 s) at 50 mg/time, 3 times/d; The course of treatment was 28 days.
Outcome measures
Primary outcome measures
Serum inflammatory factors: Fasting peripheral venous blood was extracted from each patient, followed by 15-min centrifugation (4,000 rpm). Then serum tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were quantified using enzyme-linked immunosorbent assay (ELISA) (MyBioSource Company; Lot numbers: MBS824943 and MBS2019894), and high sensitivity C-reactive protein (hs-CRP) in the serum was determined using the immunoturbidimetry assay with kit from the Beckman Coulter Company of the United States (Lot number: 378020). All these operations were conducted under strict guidelines. Granulation tissue grading: After 28 days of treatment, granulation tissue was graded and compared. Grade 0: necrosis; grade 1: edema; grade 2: pale; grade 3: aging; grade 4: healthy [13]. Efficacy: According to the following efficacy criteria, the efficacy in the two groups was analyzed and compared; Markedly effective: The symptoms disappeared after treatment, and the wound healed by 70%-100%; Effective: The symptoms were relieved and the wound healed by 30%-69%; Ineffective: After treatment, the symptoms were alleviated, and the wound healing was below 30% or the wound healing was not improved or even aggravated. Overall response rate = (the number of cases with markedly effective treatment + that of cases with effective treatment)/total cases × 100%.
Secondary outcome measures
The wound healing time, hospitalization time and the incidence of adverse reactions (including nausea and vomiting, dizziness, headache, pruritus, etc.) in the two groups were recorded and compared. Independent risk factors of poor prognosis: The prognosis of the patients within 1 year after treatment was analyzed, and the independent risk factors of poor prognosis were analyzed through logistic regression.
Statistical analyses
SPSS22 statistical software was used for statistical analyses of all the data, and GraphPad Prism 8 was used for data visualization. The counting data were described by rate, and their inter-group comparison was conducted using the chi-square test. The measurement data were described by Mean ± SD, and their inter-group comparison was conducted using the t test. Independent risk factors affecting the prognosis of patients were analyzed by logistic regression. P<0.05 suggested a notable difference.
Results
Comparison of baseline data between the two groups
The two groups were not statistically different in age, sex, body mass index (BMI), diabetes type, Wagner grade and DFI grade (all P>0.05), so the two groups were comparable (Table 1).
Table 1.
Comparison of baseline data between the two groups
| Study group (n=43) | Control group (n=37) | X2 value | P value | |
|---|---|---|---|---|
| Age | 0.288 | 0.591 | ||
| ≥50 years old | 20 | 15 | ||
| <50 years old | 23 | 22 | ||
| Gender | 0.218 | 0.641 | ||
| Male | 30 | 24 | ||
| Female | 13 | 13 | ||
| BMI | 0.244 | 0.622 | ||
| ≥23 kg/m2 | 29 | 23 | ||
| <23 kg/m2 | 14 | 14 | ||
| Diabetes mellitus type | 0.013 | 0.908 | ||
| Type I | 5 | 4 | ||
| Type II | 38 | 33 | ||
| Wagner grading | 1.014 | 0.314 | ||
| Grade II | 15 | 17 | ||
| Grade III | 28 | 20 | ||
| Classification of diabetic foot infection | 0.548 | 0.459 | ||
| Grade II | 14 | 15 | ||
| Grade III | 29 | 22 |
Note: BMI: Body mass index.
Comparison of serum inflammatory factors between the two groups
Before treatment, the two groups were not significantly different in the levels of TNF-α, hs-CRP, and IL-6 (all P>0.05), while after treatment, the levels of each decreased significantly in both groups (all P<0.05), with notably lower levels in the study group than those in the control group (P<0.05, Figure 1).
Figure 1.
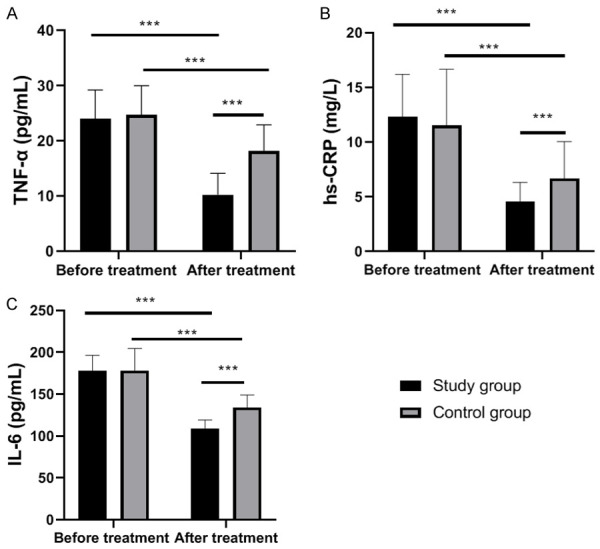
Comparison of serum inflammatory factors between the two groups. A: TNF-α level in two groups before and after treatment. B: hs-CRP level in the two groups before and after treatment. C: IL-6 level in the two groups before and after treatment. Notes: ***, P<0.001; TNF-α: tumor necrosis factor-α; hs-CRP: high sensitivity C-reactive protein; IL-6: interleukin-6.
Comparison of granulation tissue grade between the two groups
According to evaluation and comparison of the granulation tissue grade between the two groups, the study group had a notably lower proportion of patients with grade 0/grade 1 granulation tissue than the control group (P<0.05), and had a notably higher proportion of patients with grade 2/grade 4 granulation tissue than the control group (P<0.05), but the proportion of patients with grade 3 granulation tissue in the two groups was not significantly different (P>0.05, Table 2).
Table 2.
Granulation tissue grading of the two groups
| Group | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Study group (n=43) | 4 (9.30) | 6 (13.95) | 9 (20.93) | 14 (32.56) | 10 (23.26) |
| Control group (n=37) | 10 (27.02) | 15 (40.54) | 2 (5.41) | 8 (21.62) | 2 (5.41) |
| χ2 value | 4.328 | 7.262 | 4.042 | 1.193 | 4.970 |
| P value | 0.038 | 0.007 | 0.044 | 0.275 | 0.026 |
Comparison of wound healing time and hospitalization time between the two groups
According to statistical analysis on wound healing time and hospitalization time in the two groups, the study group experienced notably shorter wound healing time and hospitalization time than the control group (both P<0.05, Figure 2).
Figure 2.
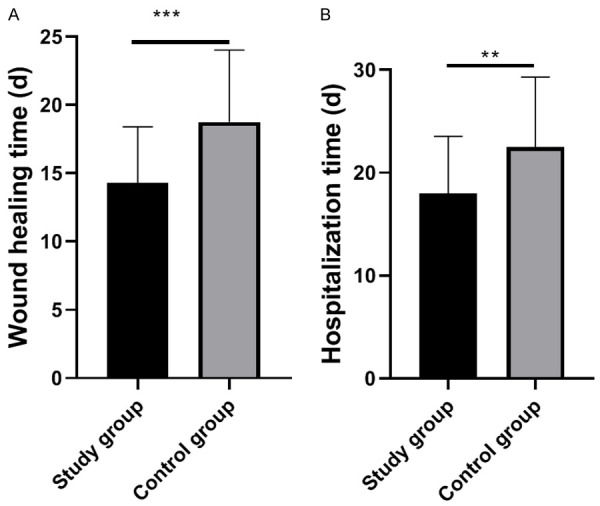
Wound healing time and hospitalization time of the two groups. A: Wound healing time of the two groups. B: Hospitalization time of the two groups. Notes: **, P<0.01; ***, P<0.001.
Efficacy in the two groups
Statistics of efficacy in the two groups revealed a notably higher overall response rate in the study group than that in the control group (P=0.030, Table 3).
Table 3.
Efficacy in the two groups
| Group | Markedly effective | Effective | Ineffective | Overall response |
|---|---|---|---|---|
| Study group (n=43) | 25 (58.14) | 15 (34.88) | 3 (6.98) | 40 (93.02) |
| Control group (n=37) | 15 (40.54) | 13 (35.14) | 9 (24.32) | 28 (75.68) |
| χ2 value | 4.694 | |||
| P value | 0.030 |
Incidence of adverse reactions in the two groups
According to statistics of adverse reactions (nausea, vomiting, dizziness, headache, pruritus, etc.) in the two groups, the study group was not greatly different from the control group in terms of the total incidence of adverse reactions (P>0.05, Table 4).
Table 4.
Incidence of adverse reactions in the two groups
| Group | Nausea and vomiting | Dizziness | Headache | Itchy | Total adverse reaction |
|---|---|---|---|---|---|
| Study group (n=43) | 2 (4.65) | 1 (2.33) | 1 (2.33) | 2 (4.65) | 6 (13.96) |
| Control group (n=37) | 4 (10.81) | 1 (2.70) | 2 (5.41) | 3 (8.11) | 10 (27.03) |
| χ2 value | 2.124 | ||||
| P value | 0.145 |
Analysis of related factors affecting prognosis
The patients were re-grouped according to the recurrence within one year after treatment. Patients with recurrence were assigned to the poor-prognosis group (n=21) and patients without recurrence were included into good-prognosis group (n=59). Then the differences in clinical data between the two groups were compared and the data were subjected to univariate analysis. According to the results, BMI, DM type, Wagner grading and classification of diabetic foot infection were the risk factors affecting the prognosis of patients (Table 5). The indicators with notable differences in the above were assigned (Table 6) and subjected to multivariate analysis. According to logistic regression analysis, Wagner grading was an independent risk factor affecting the patients’ prognosis (Table 7).
Table 5.
Univariate analysis of factors affecting prognosis
| Good-prognosis group (n=59) | Poor-prognosis group (n=21) | X2 | P value | |
|---|---|---|---|---|
| Age | 0.02147, 1 | 0.8835 | ||
| ≥50 years old | 32 | 11 | ||
| <50 years old | 27 | 10 | ||
| Gender | 0.3958, 1 | 0.5293 | ||
| Male | 30 | 9 | ||
| Female | 29 | 12 | ||
| BMI | 23.23, 1 | <0.0001 | ||
| ≥23 kg/m2 | 15 | 18 | ||
| <23 kg/m2 | 44 | 3 | ||
| Diabetes mellitus type | 21.40, 1 | <0.0001 | ||
| Type I | 10 | 15 | ||
| Type II | 49 | 6 | ||
| Wagner grading | 19.67, 1 | <0.0001 | ||
| Grade II | 48 | 6 | ||
| Grade III | 11 | 15 | ||
| Classification of diabetic foot infection | 12.60, 1 | 0.0004 | ||
| Grade II | 35 | 3 | ||
| Grade III | 24 | 18 |
Note: BMI: Body mass index.
Table 6.
Assignment of the factors
| Factors | Assignment |
|---|---|
| BMI | <23 kg/m2 =0, ≥23 kg/m2 =1 |
| Diabetes mellitus type | Type II =0, Type I =1 |
| Wagner grading | Grade II =0, grade III =1 |
| Classification of diabetic foot infection | Class II =0, class III =1 |
| Prognosis | Good prognosis =0, poor prognosis =1 |
Note: BMI: Body mass index.
Table 7.
Multivariate analysis of factors affecting prognosis
| B | S.E. | Wals | df | Sig. | Exp (B) | 95% C.I. of EXP (B) | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| BMI | 0.170 | .558 | 0.092 | 1 | 0.761 | 1.185 | 0.397 | 3.539 |
| Diabetes mellitus type | -1.182 | .693 | 2.911 | 1 | 0.088 | 0.307 | 0.079 | 1.192 |
| Wagner grading | 1.095 | .541 | 4.093 | 1 | 0.043 | 2.990 | 1.035 | 8.637 |
| Classification of diabetic foot infection | 0.317 | .548 | 0.334 | 1 | 0.563 | 1.373 | 0.469 | 4.020 |
Note: BMI: Body mass index.
Discussion
Diabetic mellitus (DM) is a common chronic clinical disease. Poor blood glucose control can easily trigger complications [14]. Diabetic foot is a serious complication of DM and the main culprit of non-traumatic amputation [15]. Diabetic foot patients are in a state of hyperglycemia, and their immune function is obviously reduced, which can easily induce bacterial infection. Infection is one of the crucial factors leading to the aggravation of diabetic foot. If the early infection is not effectively controlled, it will spread to the whole body quickly and pose a great threat to limbs [15,16]. DFI refers to foot ulcer or microcirculation ischemia caused by neuropathy and local microangiopathy at the distal end of ankle joint, accompanied by bacterial infection [17,18]. Without effective control of DFI, serious foot gangrene can occur and even lead to amputation, bringing huge pressure in life and economic burden to patients.
Broad-spectrum antibiotics are frequently adopted in the clinical treatment of DFI, which can effectively inhibit the proliferation of various pathogenic bacteria. However, because of the increasing variety of antibiotics and the diversity of pathogenic bacteria, the probability of blind drug use gradually increases, which leads to the formation of drug-resistant strains and compromises the clinical treatment effect [19,20]. Epalrestat is an aldose reductase inhibitor, which can inhibit sorbitol aggregation in red blood cells and reduce the effect of sorbitol on nerve cell function, thus achieving the effect of enhancing nerve fiber regeneration and treating diabetic foot [21]. Li et al. [22] have revealed that epalrestat can protect patients from diabetic peripheral neuropathy by reducing oxidative stress and inhibiting polyol pathway. This study investigated the efficacy of epalrestat on patients with DFI and its effects on serum inflammatory factors in the patients.
TNF-α is an inflammatory initiator, which can participate in inflammatory reactions, induce the expression of adhesion factors and promote vasculitis and can also stimulate monocytes and macrophages to secrete IL-6, thus regulating the body’s defense [23]. IL-6 is a crucial pro-inflammatory factor, which is similar to TNF-α. Its expression in large quantities in the circulation will destroy the immune balance of the body, and eventually trigger microcirculation disturbance by inducing platelet aggregation [24]. Hs-CRP is also a frequently-adopted index to evaluate the degree of inflammation [25]. In this study, the levels of inflammatory factors in the two groups were determined before and after treatment. According to the results, before treatment, the two groups were not significantly different in the levels of TNF-α, hs-CRP, and IL-6, while after treatment, the levels in both groups decreased notably, with notably lower levels in the study group than those in the control group. The results imply the ability of epalrestat to effectively alleviate the inflammatory reaction of patients with DFI. Sato et al. [26] have also revealed that epalrestat suppresses inflammatory response in lipopolysaccharide-stimulated RAW264.7 cells and targeting the regulation of pro-inflammatory cytokine levels and inflammatory mediators by epalrestat is a promising therapeutic approach to treat inflammatory injury, which strongly support the conclusion of the present study. Granulation tissue takes a crucial part in wound healing [27]. In this study, the granulation tissue grade in the two groups was evaluated and compared. The study group had a notably lower proportion of patients with grade 0/grade 1 granulation tissue than the control group, and had a notably higher proportion of patients with grade 2/grade 4 granulation tissue than the control group, but the proportion of patients with grade 3 granulation tissue in the two groups was not greatly different. The results suggest that epalrestat is conducive to granulation tissue structure and epidermal cell regeneration, and takes a strong part in wound healing. For the purpose of understanding the wound recovery and hospitalization of the two groups, the wound healing time and hospitalization time of the two groups were evaluated. According to the results, the study group experienced a significantly shorter wound healing time and hospitalization time than the control group. This shows that epalrestat is helpful to promote wound healing and shorten the hospitalization time of patients. In addition, in this study, the study group showed a notably higher overall response rate than the control group, suggesting a significant effect of epalrestat on DFI. Finally, the incidence of adverse reactions in the two groups was statistically analyzed. According to the results, the study group had no notable difference with the control group in terms of the total incidence of adverse reactions, which indicated that epalrestat is safe and effective because it would not bring more adverse reactions. According to analysis of the factors affecting the prognosis of patients, BMI, DM type, Wagner grading and classification of diabetic foot infection were the risk factors affecting the prognosis of patients. According to Logistics regression analysis, Wagner grading was an independent risk factor affecting the prognosis of patients.
This study has verified the efficacy of epalrestat on patients with DFI and its effects on serum inflammatory factors in the patients, but it still has some limitations. First of all, the limited sample size of this study may lead to some deviation in the conclusion of the study. Secondly, the patients were only followed up for one year, so their long-term prognosis is still unclear. In addition, there are various drugs for the treatment of DFI, but this study did not compare the effects of epalrestat and other drugs on DFI. Therefore, we hope to conduct a more comprehensive analysis on the effect of epalrestat on DFI based on with a larger sample size to improve the experimental conclusion.
To sum up, epalrestat can lower the levels of serum inflammatory factors, shorten the time of wound healing and hospitalization, and promote the growth and recovery of granulation in patients with DFI, without increasing adverse reactions. Thus it is worthy of clinical promotion.
Acknowledgements
Subject of Jiangxi Provincial Health and Health Committee, Subject No. 202141026.
Disclosure of conflict of interest
None.
References
- 1.Reardon R, Simring D, Kim B, Mortensen J, Williams D, Leslie A. The diabetic foot ulcer. Aust J Gen Pract. 2020;49:250–255. doi: 10.31128/AJGP-11-19-5161. [DOI] [PubMed] [Google Scholar]
- 2.Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, Game F. Diabetic foot ulcer classifications: a critical review. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3272. doi: 10.1002/dmrr.3272. [DOI] [PubMed] [Google Scholar]
- 3.Nather A, Cao S, Chen JLW, Low AY. Prevention of diabetic foot complications. Singapore Med J. 2018;59:291–294. doi: 10.11622/smedj.2018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chastain CA, Klopfenstein N, Serezani CH, Aronoff DM. A clinical review of diabetic foot infections. Clin Podiatr Med Surg. 2019;36:381–395. doi: 10.1016/j.cpm.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Riedel U, Schussler E, Hartel D, Keiler A, Nestoris S, Stege H. Wound treatment in diabetes patients and diabetic foot ulcers. Hautarzt. 2020;71:835–842. doi: 10.1007/s00105-020-04699-9. [DOI] [PubMed] [Google Scholar]
- 6.Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res. 2021;54:1080–1093. doi: 10.1021/acs.accounts.0c00864. [DOI] [PubMed] [Google Scholar]
- 7.He J, Gao HX, Yang N, Zhu XD, Sun RB, Xie Y, Zeng CH, Zhang JW, Wang JK, Ding F, Aa JY, Wang GJ. The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol Sin. 2019;40:86–97. doi: 10.1038/s41401-018-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28:646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Rivera F, Concheiro A, Alvarez-Lorenzo C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur J Pharm Biopharm. 2018;122:126–136. doi: 10.1016/j.ejpb.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Du F, Ma J, Gong H, Bista R, Zha P, Ren Y, Gao Y, Chen D, Ran X, Wang C. Microbial infection and antibiotic susceptibility of diabetic foot ulcer in China: literature review. Front Endocrinol (Lausanne) 2022;13:881659. doi: 10.3389/fendo.2022.881659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozkan S, Adanas C, Alp HH. Is ischaemia-modified albumin a biomarker in wagner classification in diabetic foot ulcers? Int J Clin Pract. 2021;75:e14830. doi: 10.1111/ijcp.14830. [DOI] [PubMed] [Google Scholar]
- 12.Bolton L. Diabetic foot ulcer: treatment challenges. Wounds. 2022;34:175–177. [PubMed] [Google Scholar]
- 13.Zhao X, Liu Z, Agu E, Wagh A, Jain S, Lindsay C, Tulu B, Strong D, Kan J. Fine-grained diabetic wound depth and granulation tissue amount assessment using bilinear convolutional neural network. IEEE Access. 2019;7:179151–179162. doi: 10.1109/access.2019.2959027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersmann A, Muller-Wieland D, Muller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127:S1–S7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 15.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–390. doi: 10.1038/s41581-020-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitocco D, Spanu T, Di Leo M, Vitiello R, Rizzi A, Tartaglione L, Fiori B, Caputo S, Tinelli G, Zaccardi F, Flex A, Galli M, Pontecorvi A, Sanguinetti M. Diabetic foot infections: a comprehensive overview. Eur Rev Med Pharmacol Sci. 2019;23:26–37. doi: 10.26355/eurrev_201904_17471. [DOI] [PubMed] [Google Scholar]
- 17.Ghotaslou R, Memar MY, Alizadeh N. Classification, microbiology and treatment of diabetic foot infections. J Wound Care. 2018;27:434–441. doi: 10.12968/jowc.2018.27.7.434. [DOI] [PubMed] [Google Scholar]
- 18.Blanchette V, Brousseau-Foley M. Multidisciplinary management of diabetic foot ulcer infection. Rev Med Interne. 2021;42:193–201. doi: 10.1016/j.revmed.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Wukich DK, Johnson MJ, Raspovic KM. Limb salvage in severe diabetic foot infection. Foot Ankle Clin. 2022;27:655–670. doi: 10.1016/j.fcl.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Saeed K, Esposito S, Akram A, Ascione T, Bal AM, Bassetti M, Carnelutti A, Chan M, Davis J, Dryden M, Farhan MFM, Fernando S, Gottlieb T, Gould I, Yildiz M, Lye DC, Pagliano P, Poole S, Pottinger PS, Spera AM, Unal S, Yalcin AN International Society of Antimicrobial Chemotherapy. Hot topics in diabetic foot infection. Int J Antimicrob Agents. 2020;55:105942. doi: 10.1016/j.ijantimicag.2020.105942. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler D, Papanas N, Schnell O, Nguyen BDT, Nguyen KT, Kulkantrakorn K, Deerochanawong C. Current concepts in the management of diabetic polyneuropathy. J Diabetes Investig. 2021;12:464–475. doi: 10.1111/jdi.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li QR, Wang Z, Zhou W, Fan SR, Ma R, Xue L, Yang L, Li YS, Tan HL, Shao QH, Yang HY. Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen Res. 2016;11:345–351. doi: 10.4103/1673-5374.177745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The role of tumor necrosis factor alpha (TNF-alpha) in autoimmune disease and current TNF-alpha inhibitors in therapeutics. Int J Mol Sci. 2021;22:2719. doi: 10.3390/ijms22052719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. 2018;10:a028456. doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Wang L, Jiang P, Kang R, Xie Y. Correlation between hs-CRP, IL-6, IL-10, ET-1, and chronic obstructive pulmonary disease combined with pulmonary hypertension. J Healthc Eng. 2022;2022:3247807. doi: 10.1155/2022/3247807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Tatsunami R, Wakame K. Epalrestat suppresses inflammatory response in lipopolysaccharide-stimulated RAW264.7 cells. Allergol Immunopathol (Madr) 2021;49:1–8. doi: 10.15586/aei.v49i5.102. [DOI] [PubMed] [Google Scholar]
- 27.Mendame Ehya RE, Zhang H, Qi B, Yu A. Application and clinical effectiveness of antibiotic-loaded bone cement to promote soft tissue granulation in the treatment of neuropathic diabetic foot ulcers complicated by osteomyelitis: a randomized controlled trial. J Diabetes Res. 2021;2021:9911072. doi: 10.1155/2021/9911072. [DOI] [PMC free article] [PubMed] [Google Scholar]


