Summary
Background
Gender-based disparities in health-care are common and can affect access to care. We aimed to investigate the impact of gender and socio-environmental indicators on health-care access in oncology in France.
Methods
Using the national health insurance system database in France, we identified patients (aged ≥18 years) who were diagnosed with solid invasive cancers between the 1st of January 2018 and the 31st of December 2019. We ensured that only incident cases were identified by excluding patients with an existing cancer diagnosis in 2016 and 2017; skin cancers other than melanoma were also excluded. We extracted 71 socio-environmental variables related to patients' living environment and divided these into eight categories: inaccessibility to public transport, economic deprivation, unemployment, gender-related wage disparities, social isolation, educational barriers, familial hardship, and insecurity. We employed a mixed linear regression model to assess the influence of age, comorbidities, and all eight socio-environmental indices on health-care access, while evaluating the interaction with gender. Health-care access was measured using absolute and relative cancer care expertise indexes.
Findings
In total, 594,372 patients were included: 290,658 (49%) women and 303,714 (51%) men. With the exception of unemployment, all socio-environmental indices, age, and comorbidities were inversely correlated with health-care access. However, notable interactions with gender were observed, with a stronger association between socio-environmental factors and health-care access in women than in men. In particular, inaccessibility to public transport (coefficient for absolute cancer care expertise index = −1.10 [−1.22, −0.99], p < 0.0001), familial hardship (−0.64 [−0.72, −0.55], p < 0.0001), social isolation (−0.38 [−0.46, −0.30], p < 0.0001), insecurity (−0.29 [−0.37, −0.21], p < 0.0001), and economic deprivation (−0.13 [−0.19, −0.07], p < 0.0001) had a strong negative impact on health-care access in women.
Interpretation
Access to cancer care is determined by a complex interplay of gender and various socio-environmental factors. While gender is a significant component, it operates within the context of multiple socio-environmental influences. Future work should focus on developing targeted interventions to address these multifaceted barriers and promote equitable health-care access for both genders.
Funding
None.
Keywords: Health-care access, Gender, Socio-environmental factors, Oncology
Research in context.
Evidence before this study
Sex, while typically characterised by biological distinctions between males and females, and gender, related to sociocultural differences between men and women, influence health-care experiences, access to care, and interactions within health-care systems. Gender-based disparities in health-care access have long been recognised as a crucial issue, particularly in oncology. We conducted a systematic search of PubMed, Google Scholar and Scopus, between database inception and June 1, 2022, using the terms (“healthcare” or “health care”) and (“sociodemographic” or “socio-demographic” or “socio-environmental”) and (“gender” or “sex”) and (“oncology” or “cancer care” or “cancerology” or “cancer”). No language restrictions were applied to the search. Our preliminary review of the literature revealed that no large-scale study has specifically evaluated the disparities in health-care access in oncology, nor the impact of gender on health-care access in oncology when considering various socio-environmental factors. The overall quality of the existing evidence was considered low, emphasising the need for further research to better understand and address these disparities.
Added value of this study
Our study addresses three main gaps in the existing literature on the impact of gender and socio-environmental indicators on health-care access in oncology. First, by using a comprehensive and exhaustive nationwide database from the French national health insurance system, our research provides a large-scale and robust analysis of patients diagnosed with solid invasive cancers. Second, we investigate the influence of a diverse range of socio-environmental factors, including inaccessibility to public transport, economic deprivation, unemployment, gender-related wage disparities, social isolation, educational barriers, familial hardship and insecurity, offering a multidimensional approach to understanding health-care access disparities. Third, our study considers the interaction between gender and these socio-environmental factors, shedding light on the unique challenges faced by women in accessing oncological care. The findings of our study highlight the importance of considering gender as a key determinant of access to cancer care and reveal significant disparities in health-care access based on socio-environmental factors, especially for women. In particular, our study uncovers the substantial negative impact of inaccessibility to public transport, familial hardship, social isolation, insecurity and economic deprivation on women's access to oncological care.
Implications of all the available evidence
Understanding the disparities in health-care access experienced by men and women, particularly in oncology, is crucial for promoting gender equality. Although our findings reveal that these socio-environmental factors do not have a substantial impact on health-care access for men, the disproportionate burden faced by women indicates that addressing these disparities is a critical concern for advancing gender equality. Continued advocacy for policies focused on driving greater equity in health-care access is essential for reducing these disparities and improving the overall health of women. Furthermore, targeted interventions to address the unique challenges faced by patients in underserved areas, such as enhancing public transportation networks, fostering partnerships between university medical centres and community clinics, and implementing telemedicine, are urgent matters for policy consideration.
Introduction
Socio-economic factors are well-established determinants of health outcomes in oncology,1 influencing aspects such as incidence and mortality rates, rates of cancer screening and prevention, and treatment outcomes. Patients with cancer from deprived areas, for instance, often experience delays in diagnosis and treatment, leading to poorer outcomes.2 It’s also been reported that individuals from rural areas have lower rates of cancer screening and higher mortality, likely due to barriers to health-care access.3 Interestingly, education levels are associated with mortality for almost all types of cancer, with lower education levels linked to higher mortality rates, particularly for cancers related to tobacco use or infection.4
Sex, while typically characterised by biological distinctions between males and females, and gender, related to sociocultural differences between men and women, influence health-care experiences, access to care, and interactions within health-care systems. Some of the organs most frequently affected by cancers, such as the prostate and gynaecologic organs, are sex-specific, and the incidence of other cancers may also differ between men and women. Only 1% of breast cancers are diagnosed in men, whereas the male-to-female ratio for head-and-neck cancers has been estimated at 3:1.5 Beyond biological factors, sociological factors related to gender may also impact health-care pathways, affecting stages of diagnosis and treatment patterns. For instance, women with lung cancer are more likely to be diagnosed at earlier stages than men with the same disease6 and to undergo surgery for locoregional disease,7 whereas the opposite pattern has been reported for bladder cancer.8
The intersection of these socio-economic factors and sex or gender could compound the disparities experienced in health-care access. For example, several studies have reported differences between men and women in the relationship between socioeconomic status and cancer risk, with men being at greater risk of developing cancer if they come from a low socioeconomic background.9 Conversely, women seem to be more vulnerable when the impact of socioeconomic status on health-care access is assessed.10
The significance of being treated in a high-volume expert centre in oncology is widely recognised, with numerous studies demonstrating that higher surgical volumes correlate with reduced complication rates, enhanced oncological outcomes, and decreased costs.11,12 Additionally, treatment at high-volume centres increases the likelihood of patients receiving care in accordance with established guidelines.13 Despite the well-documented advantages of high-volume expert centres in oncology, no studies have yet investigated potential disparities in patient access to specialised cancer care facilities based on gender.
In light of these observations, our study aims to provide a clear and comprehensive insight into the impact of gender and socio-environmental indicators on health-care access in oncology on an exhaustive nationwide database. The French national health insurance system provides comprehensive coverage of 98.8% of the French population. It includes robust and granular information related to demographics, health conditions, and health-care utilisation, thus allowing a detailed and representative examination of health-care access and outcomes in oncology. We hypothesise that women and men with different socioeconomic/demographic positions receive cancer treatment at centres with divergent levels of oncological expertise.
Methods
Patient data and selection
Data were obtained from the French national health insurance system database, which covers 98.8% of the 67 million inhabitants of France. All the medical and administrative information relating to the reimbursement of French citizens for health-care expenses in hospitals is collected and aggregated within this system. The data recorded include demographic data (gender, date of birth, geographic code of the area of residence), hospital discharge reports (diagnoses, medical and surgical procedures), and all long-term illness records (diagnosis codes and date of disease onset). We selected patients diagnosed with solid invasive cancers between the 1st January 2018 and the 31st December 2019 (Appendix 1). We ensured that only incident cases were identified by excluding patients with an existing cancer diagnosis in 2016 and 2017 from the study. Patients under 18 years of age and those with gender coding discrepancies were also excluded. Finally, skin cancers other than melanoma were excluded from the study. This study followed the STROBE guidelines for observational studies (Appendix 2) and SAGER guidelines.14
Ethics
This study was performed in accordance with institutional and ethics rules concerning research based on patient data. The study was authorised by the French data protection agency (Commission nationale de l’informatique et des libertés—CNIL, under registration number 2204867v0). No informed consent was required because the data used in the study were de-identified and re-used for research purposes, in accordance with French regulations applicable to French national health insurance system data.
Patient and tumour characteristics
The clinical features assessed in this study included gender, age, comorbid conditions and type of cancer. The French national health insurance system database traditionally records sex as a binary attribute (male/female), reflecting biological distinctions established at birth and reflected on identification documents. For the purpose of our study,14 we choose to use the nomenclature ‘gender’ to encompass not only the biological distinctions but also the social and cultural differences that typically associate with the categories of men and women. Non-binary and LGBTQ+ populations could not be assessed within the scope of this study.
Age at diagnosis was analysed as a continuous variable. Comorbid conditions were identified in the database from (i) the main, relative and associated diagnoses made during hospital stays and (ii) the medical and surgical procedures undergone during hospital stays (Appendix 3).
Absolute and relative cancer care expertise indexes
The French national health insurance system assigns a unique facility code to each health-care centre where patients receive oncological care. This code helps determine the centre's volume, defined as the annual number of patients treated for each cancer type at the centre. This outcome—centre specific volume for individual cancer types—is not novel and is a validated metric extensively employed in prior studies assessing quality of care and the influence of volume on varied cancer types.12,15,16 Recognising that cancer care expertise extends beyond volume, we gathered data from the 2019 French Annual Health Facilities Statistics (SAE) to identify centres specialised in oncology, radiotherapy, and multi-disciplinary surgery. The SAE database is a comprehensive, mandatory, and publicly available survey of all public and private hospitals in France. Conducted annually, the survey provides information on hospitals' activities, services, and staff. We selected 35 variables from the SAE, divided into four categories: oncology, radiotherapy, multidisciplinary surgery and other related activities (e.g., palliative care, medication circuit). By employing a Factor Analysis of Mixed Data (FAMD), we calculated an oncological and surgical expertise score ranging from 0 (low expertise) to 1 (high expertise) for each centre (Appendix 4). The absolute cancer care expertise index was determined by the specific volume for each cancer type, weighted by the centre's oncological and surgical expertise score. While the volume specific to each cancer type underscores the centre's specialisation and depth of experience with individual cancers, the broader oncological and surgical expertise score, derived from a broader spectrum of data including multidisciplinary surgery and palliative care, reflects the centre's comprehensive capabilities across surgical and oncological disciplines.
Simultaneously, we employed an alternative methodology using a relative cancer care expertise index. Patients with rare cancers typically experience less optimal outcomes compared to those with more prevalent malignancies, a disparity largely due to gaps in expertise and a dearth of evidence-supported guidelines.17 With the absolute cancer care expertise index, we emphasised patient volume, both to resonate with this established understanding and to reflect the current emphasis on volume in oncological literature.12,15,16 However, such a volume-focused approach might introduce an inherent bias, potentially sidelining centres proficient in addressing rare cancers. To address this potential oversight, rather than relying solely on absolute counts, the relative cancer care expertise index compares the fraction of patients treated for a specific cancer type in one centre against the total treated across all centres for this cancer type. This adjustment ensures that centres treating rare cancers are not unduly penalised for naturally lower patient numbers.
For all patients, irrespective of the oncological treatment received—whether surgery, chemotherapy, radiotherapy, or palliative care—both the absolute and relative cancer care expertise indexes were used as our primary outcomes.
Based on the geographic code of the patient's place of residence and the facility code, we also calculated the distance travelled by each patient from their home to the site of oncological treatment, in kilometres.
Socio-environmental indices
Socio-environmental data for the area of residence of the patients were collected from three official French governmental open-data websites: the National Institute of Statistics and Economic Studies (www.INSEE.fr), the Observatory of Territories (www.observatoire-des-territoires.gouv.fr) and the Open Platform for French Public Data (www.data.gouv.fr). We recovered data for 71 variables organised into eight categories: inaccessibility to public transport, gender-related wage disparities, economic deprivation (e.g., poverty rate, median income), social isolation (e.g., rate of widows or elderly people living alone), unemployment, educational barriers, familial hardship (e.g., rate of large or single-parent families) and insecurity (e.g., homicide rate). To avoid collinearity among variables measuring the same dimension and to better capture the overall effect of a dimension, we performed a Principal Component Analysis (PCA) to generate indices between 0 and 1 for each category (Appendix 5).
Statistical analysis
The study population is described in terms of frequencies for qualitative variables, or medians and interquartile ranges for quantitative variables. For enhanced clarity on distribution and to discern between-group differences, continuous variables related to initial patient characteristics also include mean, standard deviation, and effect size as measured by Cohen’s d. Comparisons were performed with Chi2 tests for qualitative variables, and Mann–Whitney or Kruskal–Wallis tests for quantitative variables. Main variables were visually described for the 15 most common cancers in the cohort.
Given that the French social security system covers approximately 98.8% of the population, absolute numbers and percentages by gender were initially employed for the primary description of the variables. In a sequential manner, the article systematically delves into patient characteristics, followed by centre characteristics and socio-environmental aspects. To ensure a more robust analysis, the main model included not only gender and socio-environmental factors, but also age, number of comorbidities, and cancer type. The selection of variables was grounded in their clinical relevance, ensuring a comprehensive representation of factors that could impact the patient journey prior to cancer diagnosis. We analysed the impact of gender and socio-environmental factors on the absolute and relative cancer care expertise indexes, by performing a mixed linear regression analysis with cancer type as a random effect. We included gender, age, number of comorbidities, and socio-environmental indices as fixed-effect variables. We also checked for interactions between these variables and patient gender. Given the right-skewed nature of the absolute and relative cancer care expertise indexes distribution (Appendix 6), we employed a cube root transformation. To validate the assumption of linearity, we have implemented residual plots with Lowess (Locally Weighted Scatterplot Smoothing) lines for each predictor. In essence, this procedure allows us to visually inspect if the relationship between the predictors and the response variable adheres to a linear pattern. We also accounted for potential multicollinearity by calculating the Variance Inflation Factor (VIF) for each predictor, ensuring that all predictors had a VIF value less than five to mitigate correlation risks. If a patient's treatment involved multiple centres, we choose to consider only the treating hospital with the highest index. No missing data were observed for gender, age, and comorbidities. Only a small percentage of the patients (n = 37,779, or 6.4%) were excluded from the multivariable analysis due to incomplete data for socio-environmental factors or the cancer care expertise index. To ensure the robustness of our results, we conducted a sensitivity analysis that included only non-sex-specific cancers. All analyses were prespecified, except for the relative cancer care expertise index, which was an ad-hoc analysis. All statistical analyses were performed with R version 4.0.3.
Role of the funding source
This study was not funded.
Results
Gender disparities in cancer type and age at diagnosis
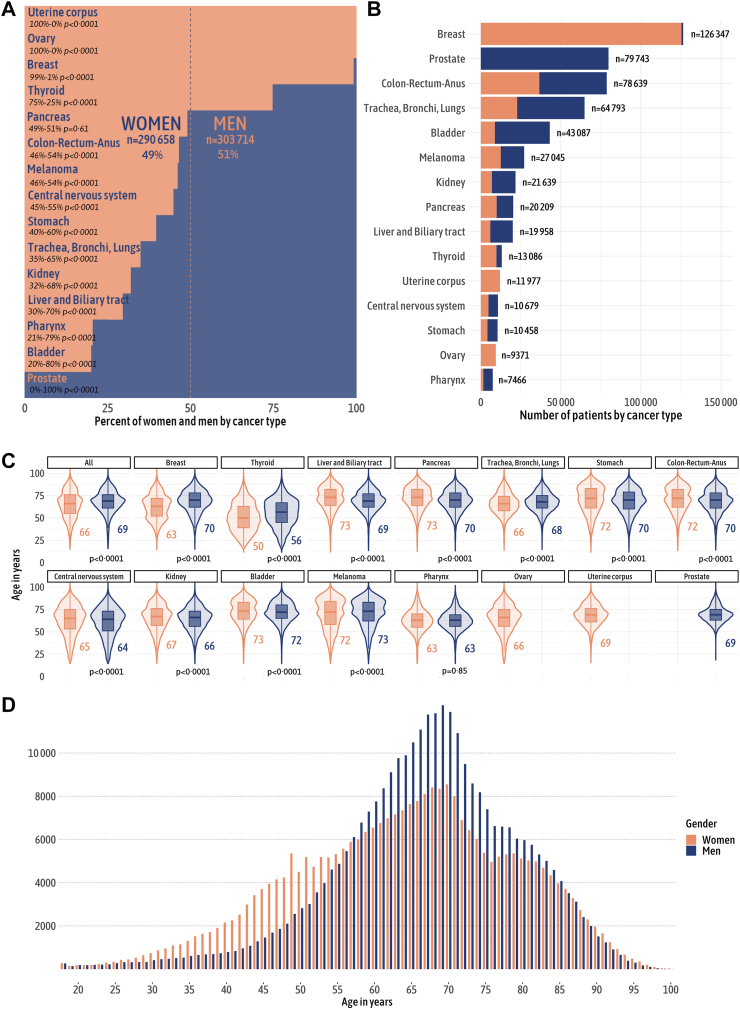
In total, 594,372 patients (women, n = 290,658, 49%; men n = 303,714, 51%) with 44 different cancer types were included in this study (Table 1). The distribution of cancers differed by gender (Appendix 7). In addition to certain sex-specific cancers, there were significant differences in cancer site distribution by gender, with breast and thyroid cancers much more frequent in women than in men, and bladder, pharynx, liver and biliary tract cancers much more common in men than in women (Fig. 1A and B). Median age at cancer diagnosis was 68 [58–76] years (66 [55–76] years in women versus 69 [61–77] years in men, p < 0.0001) (Fig. 1C) and age- and gender-related differences were clinically marked in breast (age at diagnosis of 63 [52–72] years in women versus 70 [62–78] years in men) and thyroid (age at diagnosis of 50 [39–63] years in women versus 56 [45–67] years in men) cancers. The total number of cancers was significantly higher in women than in men before the age of 55 years, whereas it was significantly higher in men than in women after the age of 55 years (Fig. 1D).
Table 1.
Overview of patient demographics, clinical features, and socio-environmental factors by gender.
| Levels | Overall | Women | Men | p valuea | Effect size (Cohen's d)b |
|---|---|---|---|---|---|
| Total number of patients | 594,372 (100%) | 290,658 (49%) | 303,714 (51%) | ||
| Patient age at diagnosis, years | |||||
| Median | Median: 68 [58–76] | 66 [55–76] | 69 [61–77] | <0.0001 | −0.21 |
| Mean | Mean: 67 (14) | 65 (15) | 68 (12) | ||
| Number of comorbidities | |||||
| 0 | 274,592 (46%) | 150,175 (52%) | 124,417 (41%) | <0.0001 | |
| 1 | 108,376 (18%) | 53,603 (18%) | 54,773 (18%) | ||
| 2–4 | 156,251 (26%) | 67,302 (23%) | 88,949 (29%) | ||
| 5+ | 55,153 (9%) | 19,578 (7%) | 35,575 (12%) | ||
| Comorbidities type | |||||
| Cardiovascular | 334,616 (56%) | 124,651 (43%) | 209,965 (69%) | <0.0001 | |
| Neuropsychiatric disorders | 147,294 (25%) | 59,989 (21%) | 87,305 (29%) | <0.0001 | |
| Endocrine | 138,295 (23%) | 66,187 (23%) | 72,108 (24%) | <0.0001 | |
| Pulmonary | 71,125 (12%) | 24,318 (8%) | 46,807 (15%) | <0.0001 | |
| Gastrointestinal | 38,948 (7%) | 16,498 (6%) | 22,450 (7%) | <0.0001 | |
| Rheumatologic disease | 15,559 (3%) | 9026 (3%) | 6533 (2%) | <0.0001 | |
| Other | 144,154 (24%) | 57,674 (20%) | 86,480 (28%) | <0.0001 | |
| Cancer type | |||||
| Breast | 126,347 (21%) | 125,288 (43%) | 1059 (0%) | <0.0001 | |
| Prostate | 79,743 (13%) | 0 (0%) | 79,743 (26%) | <0.0001 | |
| Colon-Rectum-Anus | 78,639 (13%) | 36,584 (13%) | 42,055 (14%) | <0.0001 | |
| Trachea, Bronchi, Lungs | 64,793 (11%) | 22,661 (8%) | 42,132 (14%) | <0.0001 | |
| Bladder | 43,087 (7%) | 8778 (3%) | 34,309 (11%) | <0.0001 | |
| Melanoma | 27,045 (5%) | 12,469 (4%) | 14,576 (5%) | <0.0001 | |
| Kidney | 21,639 (4%) | 6935 (2%) | 14,704 (5%) | <0.0001 | |
| Pancreas | 20,209 (3%) | 9919 (3%) | 10,290 (3%) | 0.61 | |
| Liver and Biliary tract | 19,958 (3%) | 5913 (2%) | 14,045 (5%) | <0.0001 | |
| Thyroid | 13,086 (2%) | 9774 (3%) | 3313 (1%) | <0.0001 | |
| Uterine corpus | 11,977 (2%) | 11,977 (4%) | 0 (0%) | <0.0001 | |
| Central nervous system | 10,679 (2%) | 4796 (2%) | 5883 (2%) | <0.0001 | |
| Stomach | 10,458 (2%) | 4153 (1%) | 6305 (2%) | <0.0001 | |
| Ovary | 9371 (2%) | 9371 (3%) | 0 (0%) | <0.0001 | |
| Pharynx | 7466 (1%) | 1542 (1%) | 5924 (2%) | <0.0001 | |
| Other | 49,875 (8%) | 20,498 (7%) | 29,498 (10%) | <0.0001 | |
| Social-environmental factors | |||||
| Gender-related wage disparities | Median: 0.5 [0.2–0.7] | 0.5 [0.2–0.7] | 0.5 [0.2–0.7] | <0.0001 | 0.01 |
| Mean: 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) | |||
| Economic deprivation | Median: 0.5 [0.2–0.8] | 0.5 [0.2–0.8] | 0.6 [0.2–0.8] | <0.0001 | 0.02 |
| Mean: 0.5 (0.3) | 0.5 (0.3) | 0.5 (0.3) | |||
| Educational barriers | Median: 0.4 [0.2–0.6] | 0.4 [0.2–0.6] | 0.4 [0.2–0.6] | <0.0001 | 0.05 |
| Mean: 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.3) | |||
| Inaccessibility to public transport | Median: 0.6 [0.3–0.7] | 0.6 [0.3–0.7] | 0.6 [0.3–0.7] | <0.0001 | 0.09 |
| Mean: 0.5 (0.3) | 0.5 (0.2) | 0.5 (0.2) | |||
| Unemployment | Median: 0.4 [0.1–0.8] | 0.4 [0.1–0.8] | 0.5 [0.2–0.8] | <0.0001 | 0.06 |
| Mean: 0.5 (0.3) | 0.4 (0.3) | 0.5 (0.3) | |||
| Social isolation | Median: 0.4 [0.26–0.62] | 0.4 [0.3–0.6] | 0.4 [0.3–0.6] | <0.0001 | 0.02 |
| Mean: 0.5 (0.2) | 0.4 (0.2) | 0.5 (0.2) | |||
| Familial hardship | Median: 0.5 [0.3–0.6] | 0.4 [0.3–0.6] | 0.5 [0.3–0.6] | <0.0001 | 0.04 |
| Mean: 0.5 (0.2) | 0.4 (0.2) | 0.5 (0.2) | |||
| Insecurity | Median: 0.6 [0.3–1.0] | 0.6 [0.3–1.0] | 0.6 [0.3–1.0] | <0.0001 | −0.07 |
| Mean: 0.6 (0.3) | 0.6 (0.3) | 0.6 (0.3) |
The p value indicates the statistical significance of the differences between the two genders groups for each parameter.
Cohen’s d aids in interpreting the magnitude of the difference between two groups, here women and men. An absolute value of Cohen’s d inferior to 0.20 indicates that the magnitude of the difference is minimal, even if the statistical test shows significance.
Fig. 1.
Gender-specific distribution of cancer types and age. (A) Percentage distribution of cancer types, differentiated by gender. The p value indicates the statistical significance of the differences between genders groups concerning cancer type distribution. The data reveal that men constitute the majority in most types of cancer. (B) Absolute number of cases for each cancer type, stratified by gender. Breast cancer is found to be the most common, followed by prostate and colon-rectum-anus cancers. (C) Age distribution by gender and cancer type. The outer shape represents the kernel density estimation of the age distribution, while the inner boxplot highlights the median and quartiles. The p value indicates the statistical significance of the differences between genders groups concerning age distribution by cancer type. Our findings indicate significant clinical differences in the age of diagnosis for breast, thyroid, and liver and biliary tract cancers between men and women. (D) Age distribution by gender reveals that cancers at a young age are more prevalent in women, while men form the majority of cases after the age of 55.
Gender-based differences in comorbidity profiles at cancer diagnosis
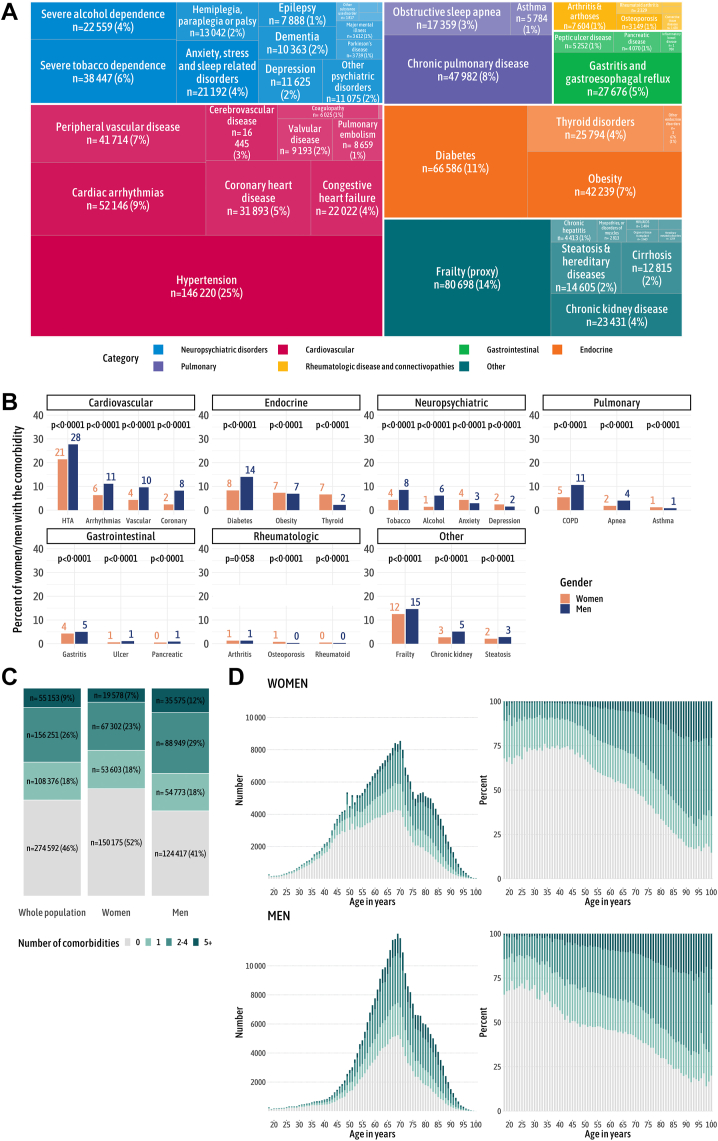
Overall, 179,297 (59%) men and 140,483 (48%) women had at least one comorbid condition at diagnosis. The three most frequent comorbid conditions were hypertension (affecting 146,220 patients, or 25%), diabetes (66,585, or 11%) and cardiac arrhythmia (52,146, or 9%). Additionally, frailty was identified in 80,698 patients, constituting 14% of the cohort (Fig. 2A). The frequency of comorbid conditions differed significantly by gender (Fig. 2B), with a higher prevalence of comorbid conditions in men than in women, except for rheumatologic diseases, as the prevalence of osteoporosis and rheumatoid arthritis was higher in women. In the overall population, 274,592 (46%) patients were free from comorbid conditions at diagnosis (Fig. 2C). Comorbidity profiles by age differed significantly by gender, with notably higher frequencies and numbers of comorbid conditions in men than in women (Fig. 2D).
Fig. 2.
Comorbidity patterns in the study population. (A) Overall distribution of comorbidity types. The data show that hypertension, diabetes, and cardiac arrhythmias are the most common comorbidities, with frailty present in 14% of patients at diagnosis. (B) Distribution of comorbidity types, separated by gender. The p value represents the statistical significance of the differences between genders groups concerning comorbidity types distribution. Men exhibit a higher prevalence of comorbid conditions, with the exception of rheumatologic diseases and anxiodepressive disorders. (C) Number of comorbidities in the entire population and by gender, indicating that 59% of men versus 48% of women present at least one comorbid condition at the time of cancer diagnosis. (D) The number of comorbidities by age and gender, demonstrating that men consistently show a higher number of comorbidities at diagnosis across all age groups.
Gender-specific variations in characteristics of cancer treatment centres
This study included 1448 facilities that treated at least one cancer patient between 2018 and 2019. The analysis of annual centre volume reveals a majority of low-volume centres across all cancer types (Appendix 8A). The median annual volume per centre varied substantially among cancer types, with central nervous system cancer having the lowest at 2 [1–7] and colon cancer the highest at 32 [6–71]. Women more often received oncological treatment at higher volume centres for breast cancer (262 [122–607] compared to 228 [93–554] for men, p < 0.0001), while men were more likely to be treated at higher volume centres for liver and biliary tract cancer (50 [17–150] compared to 31 [13–117] for women, p < 0.0001, Appendix 8B). Among the included facilities, 690 (49%) lacked authorisation for cancer surgery, 744 (52%) did not offer chemotherapy services, and 1293 (91%) did not provide radiotherapy activities (Appendix 8C). Complete data from the SAE database were missing for 29 (2%) facilities. The centres' median oncological expertise score was 0.3, with a range of 0.1 to 0.8 (Appendix 8D). The median distance to the cancer centre was 21 [8–45] kilometres for both genders (Appendix 8E). The analysis of distance-to-centre by cancer site revealed that the distance travelled was slightly greater in men than in women for several cancer types, in particular stomach (14 [5–33] kilometres for women versus 18 [7–38] for men, p < 0.0001), liver and biliary tract (18 [7–44] versus 22 [8–52], p < 0.0001), central nervous system (25 [10–55] versus 28 [11–55], p < 0.0001), and pharynx (22 [7–50] versus 25 [10–55], p < 0.0001). The distribution of the cancer care expertise indexes among centres can be visualised in Appendix 8F and G.
Gender discrepancies in socio-environmental indices
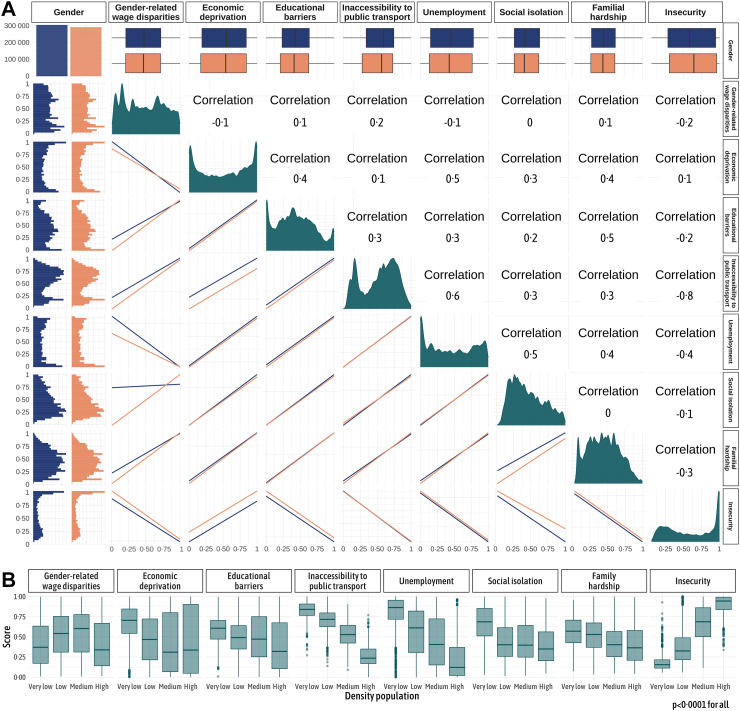
For each patient, we calculated socio-environmental indices, including gender-related wage disparities, economic deprivation, educational barriers, inaccessibility to public transport, unemployment, social isolation, familial hardship and insecurity (Fig. 3A). Higher scores, nearing 1, indicate a more disadvantaged environment for the patient. Although we did not detect multicollinearity (VIF inferior than 5), the correlation analysis between the socio-environmental scores reveals a concurrent trend: when the score for economic deprivation increases, the scores for educational barriers, inaccessibility to public transport, unemployment, social isolation, and familial hardship tend to rise as well, reflecting the interlinked nature of these socio-environmental factors (Pearson correlation coefficients between 0 and 0.6). The correlation between the gender-related wage disparities index and other indices is less straightforward, with a negative correlation noted with both unemployment (Pearson correlation coefficient −0.1) and economic deprivation (Pearson correlation coefficient −0.1). The insecurity index also exhibited a complex relationship, correlating positively with economic deprivation (Pearson correlation coefficient 0.1), but inversely with the majority of other indices, especially public transport inaccessibility (Pearson correlation coefficient-0.8). Except for insecurity index, socio-environmental indicators were generally less favourable in rural areas compared to urban areas (Fig. 3B, Appendix 9). However, notable disparities were also evident within metropolitan areas, as exemplified by the economic deprivation scores, ranging from 0.2 to 0.8 in high-density areas.
Fig. 3.
Assessment of socio-environmental factors in the study cohort. (A) Distribution of socio-environmental factors in the total population and by gender, complemented by a Pearson correlation analysis between the socio-environmental scores. The first column and first row manifest the gender-specific distribution of the scores, with a histogram to capture the complete distribution and a box plot to succinctly portray the median. The dark green diagonal encapsulates the overall distribution of the socio-environmental scores. The remaining panels depict pairwise correlations among all eight socio-environmental scores. The correlation analysis underscores a substantial interrelation among economic deprivation, educational barriers, inaccessibility to public transport, unemployment, social isolation, and familial hardship scores, as reflected by Pearson correlation coefficients spanning from 0 to 0.6. This suggests that when the economic deprivation score increases, the scores for educational barriers, public transport inaccessibility, unemployment, social isolation, and familial hardship also rise, indicating their linked progression. (B) Relation between socio-environmental scores and population density. These boxplots display the median and interquartile range (IQR), with whiskers extending up to 1.5 times the IQR from the box, and individual points beyond the whiskers indicating potential outliers. The p-value represents the statistical significance of differences in the distribution of socio-environmental factors among population density groups. Socio-environmental indicators generally show less favourable scores in rural than in urban areas, with the exception of the insecurity score. Notably, significant disparities are also observed within metropolitan regions, as exemplified by the economic deprivation scores.
Multivariable model for cancer care expertise indexes
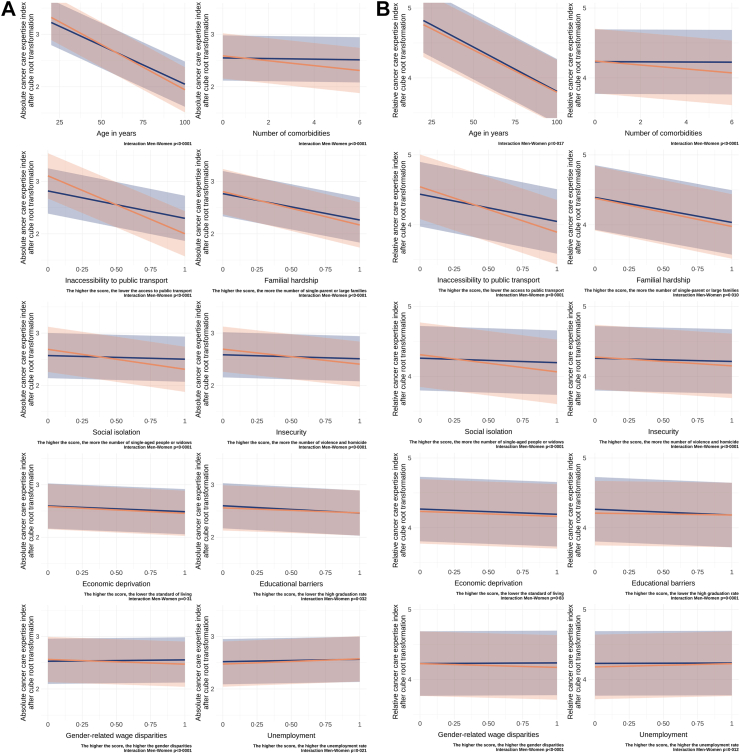
Together with increasing age and number of comorbid conditions, all socio-environmental indices, except for unemployment, were significantly negatively associated with the absolute and relative cancer care expertise indexes (Table 2, Fig. 4A and B). However, strong interactions with gender were detected, indicating a more pronounced association between socio-environmental factors and health-care access in women compared to men. The socio-environmental factors with the most substantial influence on access to specialised cancer care, especially for women, included inaccessibility to public transport (coefficient for women for absolute cancer care expertise index = −1.10 [−1.22, −0.99], p < 0.0001), familial hardship (−0.64 [−0.72, −0.55], p < 0.0001), social isolation (−0.38 [−0.46, −0.30], p < 0.0001), insecurity (−0.29 [−0.37, −0.21], p < 0.0001), and economic deprivation (−0.13 [−0.19, −0.07], p < 0.0001). Qualitatively similar findings were observed for the relative cancer care expertise index, though some significance levels were modified.
Table 2.
Results for the multivariable mixed linear regression for absolute and relative cancer care expertise indexes.
| Absolute cancer care expertise index after cube root transformation |
Relative cancer care expertise index after cube root transformation |
|||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p valuea | Interaction men-womenb | Coefficient | 95% CI | p valuea | Interaction men-womenb | |
| Gender | p < 0.0001 | p < 0.0001 | ||||||
| Men | 4.19 | [3.76, 4.62] | p < 0.0001 | 5.57 | [5.10, 6.03] | p < 0.0001 | ||
| Women | 5.06 | [4.55, 5.56] | p < 0.0001 | 5.81 | [5.30, 6.33] | p < 0.0001 | ||
| Age, years | p < 0.0001 | 0.017 | ||||||
| Men | −0.01 | [−0.02, −0.01] | p < 0.0001 | −0.01 | [−0.01, −0.01] | p < 0.0001 | ||
| Women | −0.02 | [−0.02, −0.02] | p < 0.0001 | −0.01 | [−0.01, −0.01] | p < 0.0001 | ||
| Number of comorbidities | p < 0.0001 | p < 0.0001 | ||||||
| Men | −0.01 | [−0.01, 0.00] | 0.0001 | 0.00 | [0.00, 0.00] | 0.24 | ||
| Women | −0.05 | [−0.05, −0.04] | p < 0.0001 | −0.03 | [−0.03, −0.02] | p < 0.0001 | ||
| Inaccessibility to public transport | p < 0.0001 | p < 0.0001 | ||||||
| Men | −0.52 | [−0.57, −0.47] | p < 0.0001 | −0.39 | [−0.42, −0.36] | p < 0.0001 | ||
| Women | −1.10 | [−1.22, −0.99] | p < 0.0001 | −0.65 | [−0.73, −0.57] | p < 0.0001 | ||
| Familial hardship | p < 0.0001 | 0.010 | ||||||
| Men | −0.50 | [−0.54, −0.47] | p < 0.0001 | −0.36 | [−0.38, −0.33] | p < 0.0001 | ||
| Women | −0.64 | [−0.72, −0.55] | p < 0.0001 | −0.40 | [−0.46, −0.34] | p < 0.0001 | ||
| Social isolation | p < 0.0001 | p < 0.0001 | ||||||
| Men | −0.07 | [−0.10, −0.04] | p < 0.0001 | −0.06 | [−0.09, −0.04] | p < 0.0001 | ||
| Women | −0.38 | [−0.46, −0.30] | p < 0.0001 | −0.25 | [−0.30, −0.19] | p < 0.0001 | ||
| Insecurity | p < 0.0001 | p < 0.0001 | ||||||
| Men | −0.08 | [−0.11, −0.04] | p < 0.0001 | −0.05 | [−0.07, −0.02] | 0.0001 | ||
| Women | −0.29 | [−0.37, −0.21] | p < 0.0001 | −0.13 | [−0.18, −0.07] | p < 0.0001 | ||
| Economic deprivation | 0.31 | 0.83 | ||||||
| Men | −0.11 | [−0.13, −0.08] | p < 0.0001 | −0.07 | [−0.09, −0.06] | p < 0.0001 | ||
| Women | −0.13 | [−0.19, −0.07] | p < 0.0001 | −0.07 | [−0.12, −0.03] | p < 0.0001 | ||
| Educational barriers | 0.032 | 0.0001 | ||||||
| Men | −0.14 | [−0.17, −0.11] | p < 0.0001 | −0.08 | [−0.10, −0.06] | p < 0.0001 | ||
| Women | −0.10 | [−0.16, −0.03] | 0.0001 | −0.03 | [−0.07, 0.02] | 0.13 | ||
| Gender-related wage disparities | p < 0.0001 | p < 0.0001 | ||||||
| Men | 0.03 | [0.00, 0.05] | 0.026 | 0.01 | [−0.01, 0.03] | 0.25 | ||
| Women | −0.09 | [−0.15, −0.03] | p < 0.0001 | −0.05 | [−0.09, −0.01] | 0.0002 | ||
| Unemployment | 0.021 | 0.012 | ||||||
| Men | 0.05 | [0.01, 0.08] | 0.0051 | 0.01 | [−0.02, 0.03] | 0.64 | ||
| Women | 0.10 | [0.02, 0.18] | 0.0005 | 0.05 | [−0.01, 0.10] | 0.020 | ||
Missing data: Gender n = 0 (0%), Age n = 0 (0%), Comorbidities n = 0 (0%), Inaccessibility to public transport n = 33,122 (5.4%), Gender-related wage disparities n = 18,687 (3.1%), Familial hardship n = 26,825 (4.4%), Social isolation n = 18,609 (3.4%), Economic deprivation n = 22,257 (3.6%), Unemployment n = 18,609 (3.1%), Insecurity n = 33,396 (5.5%), Educational barriers n = 18,609 (3.0%).
CI: Confidence interval.
The p value demonstrates the significance of the relationships between the given social determinant and the cancer care expert index for each gender separately.
The p value indicates the significance of the interaction between men and women for each factor on the cancer care expertise indices.
Fig. 4.
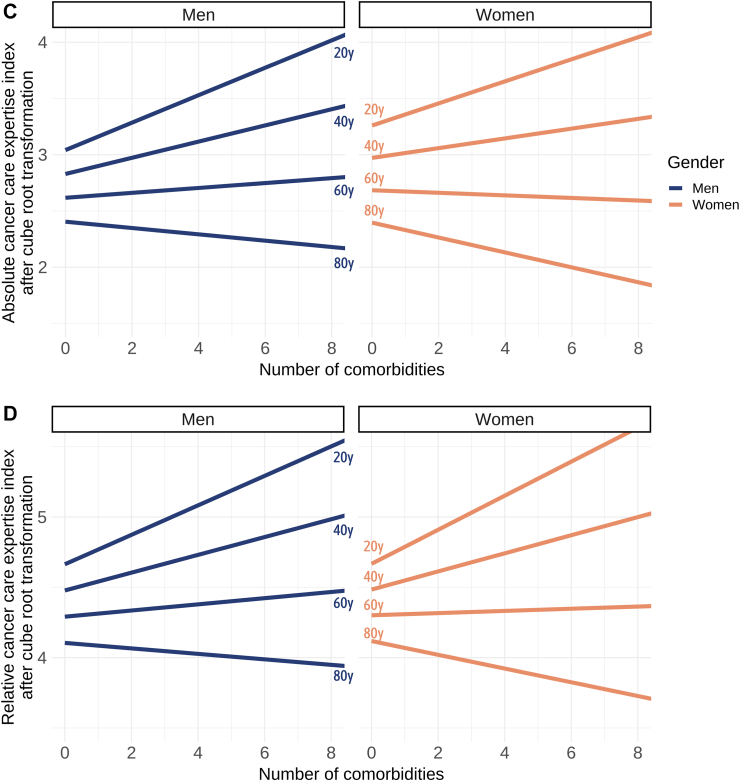
Results of multivariable analyses on the cancer care expertise indexes. (A) Results for the multivariable analyses for absolute cancer care expertise index, and (B) for relative cancer care expertise index. The p value indicates the significance of the interaction between men and women for each factor on the cancer care expertise indices. The data reveals that, except for unemployment, all calculated socio-environmental factors were negatively associated with cancer care expertise indexes. However, this association was more pronounced for women. The data underscore that the inaccessibility to public transport had the most substantial impact on health-care access. (C) Interaction between age, number of comorbidities, and gender on the absolute cancer care expertise index, and (D) on the relative cancer care expertise index. As age and the number of comorbidities increased, a more rapid shift towards treatment in lower-volume centres was observed among women compared to men.
In the multivariable analysis, a highly significant interaction was observed between gender, age, and the number of comorbid conditions. As age and the number of comorbidities increased, the decline in the cancer care expertise indexes was more pronounced for women compared to men (Fig. 4C and D).
All the outcomes of the sensitivity analysis were close to those of the main analysis (Appendix 10) and confirmed the high impact of socio-environmental factors on health-care access, particularly in women. When focusing solely on non-sex specific cancers, the sensitivity analysis demonstrated an even sharper negative impact in women than in men from economic deprivation (−0.14 [−0.21, −0.06] for women versus −0.05 [−0.08, −0.01] for men for absolute cancer care expertise index, p < 0.0001) and educational barriers (−0.12 [−0.20, −0.04] versus −0.03 [−0.06, 0.01], p = 0.0001).
Discussion
In this nationwide study in France, we investigated the effects of gender, age, comorbid conditions, and socio-environmental factors on health-care access in oncology. We identified considerable disparities in accessing specialised cancer care for patients from diverse socio-demographic backgrounds, with particularly significant gender-related differences observed among women.
In our main results, all socio-environmental factors, except for unemployment, were negatively associated with access to an oncological expert centre. However, this association was stronger for women than for men. Our findings indicate that inaccessibility to public transport had the greatest impact on health-care access for women. A study of household car ownership in urban and rural areas based on Swedish register data found that only 30% of women owned a car, whereas 53% of men were car owners.18 Women tend to use public transport more frequently than men for both work and leisure purposes, resulting in a greater dependence on such modes of transportation.19 In urban areas, high crime rates significantly limit women's mobility due to heightened concerns about physical or sexual violence, especially in public spaces and while using public transportation.20 As shown in our study, this pervasive sense of insecurity can severely restrict women's access to crucial services, including health care, by deterring them from walking alone or using public modes of transport. Our findings also highlight the greater impact of economic instability and familial hardship on access to health care in women than in men. Women are more likely to be employed in low-paid jobs and to have caregiving responsibilities constraining their ability to work full-time and to seek medical care.21 Social isolation is also more prevalent in women than in men, particularly among older women and widows, due to the gender-based disparity in life expectancy. While both genders face negative health outcomes associated with loneliness,22,23 older women, often living alone or facing the challenges of widowhood, are particularly susceptible to the adverse effects of isolation, manifesting in reduced physical activity, increased health risks, and a heightened sense of loneliness compared to their male counterparts.24,25 These social and economic barriers can result in women delaying or forgoing necessary medical care, or, as suggested by our study, may result in women travelling to the nearest health-care centre regardless of its expertise. Measuring the effect of gender has traditionally been epidemiologically challenging, due to the interconnectedness of this factor with other socio-environmental factors and the difficulty of isolating its individual impact. Here, for the first time, by including 71 socio-environmental variables grouped into eight different categories, we were able to isolate the effect of gender and of each of the individual socio-environmental determinants to obtain clear insight into their interaction and its effect on health-care access.
Centre volume is a major determinant of care quality in oncology and serves as a valuable metric for identifying disparities in health-care access. In our study, we improved this quality care outcome by weighted it by an oncological and surgical expertise score. National Comprehensive Cancer Network (NCCN) guidelines recommend surgery at high-volume facilities for rare and/or complex cancers, such as cancers of the pancreas and oesophagus, but a recent study on twelve solid cancers revealed that patients treated surgically at high-volume facilities consistently had improved overall survival when compared with those treated at low-volume centres.15 Several studies have suggested that women with various types of cancer may be undertreated in clinical practice, partially accounting for their specific survival being lower than that in men. For instance, women with head-and-neck,26 advanced bladder27 or colorectal28 cancers have been shown to be less likely to receive intensive chemoradiotherapy than men. Receiving treatment at a low-volume centre may also limit access to research and innovation, resulting in fewer opportunities for women to participate in clinical trials. Men are almost twice as likely to be included in clinical trials as women,29 particularly for elderly women and those living in very poor areas.30
The relationships of comorbid conditions with cancer risk and survivorship have been studied in detail, but little is known about the association between gender, comorbid conditions and health-care access in patients with cancer. Comorbid conditions have a disproportionately negative impact on disadvantaged populations31,32 and influence cancer diagnosis, tumour biology, and subsequent treatment choices.33, 34, 35 While socio-environmental factors primarily drive the impact of comorbidities on health-care access, these conditions may directly lead to health-care access difficulties36 and prolonged times to cancer diagnosis.37 In our study, the higher burden of comorbid conditions was much higher in men than in women. Men generally tend to have higher rates of risk factors, such as smoking, excessive alcohol consumption, and physical inactivity,38 which may contribute to a higher prevalence of comorbid conditions. However, in our multivariable model, comorbid conditions had a stronger impact on access to oncology care in women than in men. Elderly women with comorbid conditions were much more likely than men to be referred to non-expert facilities. This gender-specific difference in referral patterns has never been previously documented, but could have a considerable impact. The oncological management of elderly patients with frailties can be more challenging and may necessitate the expertise offered by high-volume centres.39,40
While this study offers valuable insights into gender disparities in health-care access for oncology patients, it is critical to address the limitations concerning the representation of gender. In the French national health insurance system database, gender is traditionally recorded as a binary attribute (male/female) based on biological sex. Our study has used this data and referred to it under the nomenclature ‘gender’ to broadly represent the societal roles and cultural differences associated with men and women. However, this binary categorisation inherently fails to capture the diversity in gender identities that fall outside the traditional male/female classification, such as non-binary individuals or members of the LGBTQ+ community. This limitation can lead to an oversimplification and may not reflect the complete spectrum of gender experiences and the unique challenges faced by individuals with non-traditional gender identities in accessing health care.
Multilevel strategies could be developed to increase access to care, particularly in rural areas where socio-environmental indicators tend to be less favourable. Such initiatives could include: i) improving public transportation options to health-care facilities, ii) fostering partnerships between university medical centres and community clinics, iii) implementing telemedicine technologies or mobile health clinics to provide remote consultations and medical services, and iv) implementing community-driven programs to combat social isolation and familial hardships. Efforts to integrate sex and gender into medical research, practice, and education are urgently needed, as the current lack of appreciation of sex- and gender-related differences is detrimental to both women and men. Our results reveal the gender of the patient influences the behaviour of both patients and clinicians and should be considered a major determinant of how, when, and why the individual receives oncological care.
Contributors
FJ ASH FR FL CA conceived the study. All authors designed the study. FJ and ASH accessed and verified the underlying data and conducted the data analysis. All authors had full access to the data in the study. All authors interpreted the results. FJ ASH FR FL wrote the manuscript and compiled all tables and figures. All authors contributed to drafts, approved the final version, and were responsible for the decision to submit the manuscript for publication.
Data sharing statement
The data that support the findings of this study are not publicly available due to the conditions of data access to the French national health insurance system database. Interested individuals can apply to the ATIH (https://www.atih.sante.fr) or Health Data Hub (https://www.health-data-hub.fr), and once approved, can apply to the corresponding author.
Declaration of interests
We declare no competing interests.
Footnotes
Translation For the French translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102298.
Appendix A. Supplementary data
References
- 1.Bongaarts J., Woolf S.H., Aron L. U.S. Health in International perspective: shorter lives, poorer health. Popul Dev Rev. 2013;39:165–167. [PubMed] [Google Scholar]
- 2.Lyratzopoulos G., Neal R.D., Barbiere J.M., Rubin G.P., Abel G.A. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13:353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 3.Henley S.J. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties — United States. MMWR Surveill Summ. 2017;66:1–13. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccarella S., Georges D., Bray F., et al. Socioeconomic inequalities in cancer mortality between and within countries in Europe: a population-based study. Lancet Reg Health Eur. 2023;25 doi: 10.1016/j.lanepe.2022.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung H., Ferlay J., Siegel R.L., et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Tolwin Y., Gillis R., Peled N. Gender and lung cancer-SEER-based analysis. Ann Epidemiol. 2020;46:14–19. doi: 10.1016/j.annepidem.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Rana R.H., Alam F., Alam K., Gow J. Gender-specific differences in care-seeking behaviour among lung cancer patients: a systematic review. J Cancer Res Clin Oncol. 2020;146:1169–1196. doi: 10.1007/s00432-020-03197-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohn J.A., Vekhter B., Lyttle C., Steinberg G.D., Large M.C. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: a nationwide claims-based investigation. Cancer. 2014;120:555–561. doi: 10.1002/cncr.28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merletti F., Galassi C., Spadea T. The socioeconomic determinants of cancer. Environ Health. 2011;10(Suppl 1):S7. doi: 10.1186/1476-069X-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou J.Y., Waters A.R., Kaddas H.K., et al. Financial burdens during the COVID-19 pandemic are related to disrupted healthcare utilization among survivors of adolescent and young adult cancers. J Cancer Surviv. 2022;17(6):1571–1582. doi: 10.1007/s11764-022-01214-y. published online May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooiker G.A., Lemmens V.E.P.P., Besselink M.G., et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg. 2014;101:1000–1005. doi: 10.1002/bjs.9468. [DOI] [PubMed] [Google Scholar]
- 12.Dikken J.L., van Sandick J.W., Allum W.H., et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg. 2013;100:83–94. doi: 10.1002/bjs.8966. [DOI] [PubMed] [Google Scholar]
- 13.Bristow R.E., Palis B.E., Chi D.S., Cliby W.A. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118:262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Heidari S., Babor T.F., De Castro P., Tort S., Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoltzfus K.C., Shen B., Tchelebi L., et al. Impact of facility surgical volume on survival in patients with cancer. J Natl Compr Cancer Netw. 2021;19:495–503. doi: 10.6004/jnccn.2020.7644. [DOI] [PubMed] [Google Scholar]
- 16.Bristow R.E., Chang J., Ziogas A., Randall L.M., Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403–410. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Gatta G., van der Zwan J.M., Casali P.G., et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Pyddoke R., Creutzer C. CTS - Centre for Transport Studies Stockholm; 2014. Household car ownership in urban and rural areas in Sweden 1999–2008.http://urn.kb.se/resolve?urn=urn:nbn:se:vti:diva-7366 [Google Scholar]
- 19.Roos J.M., Sprei F., Holmberg U. Sociodemography, geography, and personality as determinants of car driving and use of public transportation. Behav Sci (Basel) 2020;10:93. doi: 10.3390/bs10060093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauvin L., Tizzoni M., Piaggesi S., et al. Gender gaps in urban mobility. Hum Soc Sci Commun. 2020;7:1–13. [Google Scholar]
- 21.National Women’s Law Center (NWLC) The wage gap: the who, how, why, and what to do. https://nwlc.org/wp-content/uploads/2019/09/Wage-Gap-Who-how.pdf
- 22.Schrempft S., Jackowska M., Hamer M., Steptoe A. Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health. 2019;19:74. doi: 10.1186/s12889-019-6424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steptoe A., Shankar A., Demakakos P., Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagan R. Gender and age differences in loneliness: evidence for people without and with disabilities. Int J Environ Res Public Health. 2020;17:9176. doi: 10.3390/ijerph17249176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golaszewski N.M., LaCroix A.Z., Godino J.G., et al. Evaluation of social isolation, loneliness, and cardiovascular disease among older women in the US. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.46461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park A., Alabaster A., Shen H., Mell L.K., Katzel J.A. Undertreatment of women with locoregionally advanced head and neck cancer. Cancer. 2019;125:3033–3039. doi: 10.1002/cncr.32187. [DOI] [PubMed] [Google Scholar]
- 27.Rose T.L., Deal A.M., Nielsen M.E., Smith A.B., Milowsky M.I. Sex disparities in use of chemotherapy and survival in patients with advanced bladder cancer. Cancer. 2016;122:2012–2020. doi: 10.1002/cncr.30029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulson E.C., Wirtalla C., Armstrong K., Mahmoud N.N. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. 2009;52:1982–1991. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 29.Lee E., Wen P. Gender and sex disparity in cancer trials. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross C.P., Filardo G., Mayne S.T., Krumholz H.M. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–491. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 31.Agborsangaya C.B., Lau D., Lahtinen M., Cooke T., Johnson J.A. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health. 2012;12:201. doi: 10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiøtz M.L., Stockmarr A., Høst D., Glümer C., Frølich A. Social disparities in the prevalence of multimorbidity - a register-based population study. BMC Public Health. 2017;17:422. doi: 10.1186/s12889-017-4314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarfati D., Koczwara B., Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66:337–350. doi: 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 34.Panigrahi G., Ambs S. How comorbidities shape cancer biology and survival. Trends Cancer. 2021;7:488–495. doi: 10.1016/j.trecan.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renzi C., Kaushal A., Emery J., et al. Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat Rev Clin Oncol. 2019;16:746–761. doi: 10.1038/s41571-019-0249-6. [DOI] [PubMed] [Google Scholar]
- 36.Rayman G., Akpan A., Cowie M., et al. Managing patients with comorbidities: future models of care. Future Healthc J. 2022;9:101–105. doi: 10.7861/fhj.2022-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majano S.B., Lyratzopoulos G., Rachet B., de Wit N.J., Renzi C. Do presenting symptoms, use of pre-diagnostic endoscopy and risk of emergency cancer diagnosis vary by comorbidity burden and type in patients with colorectal cancer? Br J Cancer. 2022;126:652–663. doi: 10.1038/s41416-021-01603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokdad A.H., Marks J.S., Stroup D.F., Gerberding J.L. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 39.Chen R.C., Royce T.J., Extermann M., Reeve B.B. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin Radiat Oncol. 2012;22:265–271. doi: 10.1016/j.semradonc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Vitale S.G., Capriglione S., Zito G., et al. Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet. 2019;299:299–315. doi: 10.1007/s00404-018-5006-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.