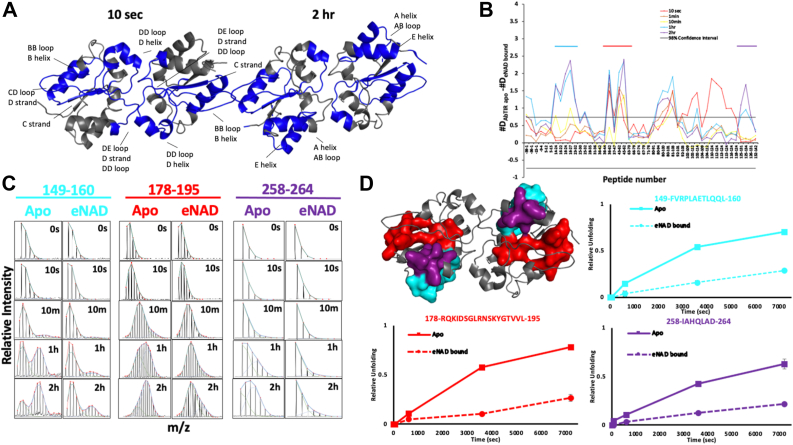
Figure 4.
HDX-MS of AbTir-TIRwtexhibit EX1 kinetics and conformations upon binding NAD+. Substrate binding results in large decreases in deuterium uptake in AbTir-TIRwt. Significant EX1 exchange kinetics consistent with large conformational changes are observed for AbTir-TIRwt, in the absence of NAD and weakened upon ligand binding. A, regions with significant decreases in deuterium uptake after 10s and 2 h incubation are mapped on the structure of AbTir-TIR. B, the difference plot (Apo – εNAD-bound) in absolute deuterium uptake at each incubation time point (color coded) and for each peptide (x-axis). C, stacked spectra of representative spectra showing the signature bimodal isotopic envelops of EX1 kinetic regime of exchange. Apo- and NAD-bound states are shown. Peptides are color coded as in panel D. D, surface and ribbon representation of AbTir-TIRwt. Surface representation shows peptides displaying bimodal EX1 behavior. Plots show the progressive accumulation of the high m/z species deconvolved from the bimodal isotopic envelop as a function of time of deuterium incubation for the apo- (solid) and εNAD-bound (dashed) AbTir-TIRwt. εNAD, ethano-NAD; AbTir-TIR, Acinetobacter baumannii TIR domain protein; HDX-MS, hydrogen-deuterium exchange mass spectrometry.