Abstract
Rationale & Objective
Innovative models are needed to address significant gaps in kidney care follow-up for acute kidney injury (AKI) survivors.
Study Design
This quasi-experimental pilot study reports the feasibility of the AKI in Care Transitions (ACT) program, a multidisciplinary approach to AKI survivor care based in the primary care setting.
Setting & Participants
The study included consenting adults with stage 3 AKI discharged home without dialysis.
Interventions
The ACT intervention included predischarge education from nurses and coordinated postdischarge follow-up with a primary care provider and pharmacist within 14 days. ACT was implemented in phases (Usual Care, Education, ACT).
Outcomes
The primary outcome was feasibility. Secondary outcomes included process and clinical outcomes.
Results
In total, 46 of 110 eligible adults were enrolled. Education occurred in 18/18 and 14/15 participants in the Education and ACT groups, respectively. 30-day urine protein evaluation occurred in 15%, 28%, and 87% of the Usual Care, Education, and ACT groups, respectively (P < 0.001). Cumulative incidence of provider (primary care or nephrologist) and laboratory follow-up at 14 and 30 days was different across groups (14 days: Usual care 0%, Education 11%, ACT 73% [P < 0.01]; 30 days: 0%, 22%, and 73% [P < 0.01]). 30-day readmission rates were 23%, 44%, and 13% in the Usual Care, Education, and ACT groups, respectively (P = 0.13).
Limitations
Patients were not randomly assigned to treatment groups. The sample size limited the ability to detect some differences or perform multivariable analysis.
Conclusions
This study demonstrated the feasibility of multidisciplinary AKI survivor follow-up beginning in primary care. We observed a higher cumulative incidence of laboratory and provider follow-up in ACT participants.
Trial Registration
Plain-Language Summary
Abrupt loss of kidney function in hospitalized patients, acute kidney injury (AKI), increases the chances of long-term kidney disease and a worse health care experience for patients. One out of 3 people who experience AKI do not get the follow-up kidney care they need. We performed a pilot study to test whether a program that facilitates structured AKI follow-up in primary care called the AKI in Care Transitions (ACT) program was possible. ACT brings together the unique expertise of nurses, doctors, and pharmacists to look at the patient’s kidney health plan from all angles. The study found that the ACT program was possible and led to more complete kidney care follow-up after discharge than the normal approach to care.
Index Words: Acute kidney injury, acute kidney injury, chronic kidney disease, care transitions, primary care, pharmacist, patient care team
Acute kidney injury (AKI) affects at least 1 in 5 hospitalized patients and puts survivors at increased risk for poor short- and long-term health outcomes, including hospital readmissions, cardiovascular events, chronic kidney disease (CKD), reduced quality of life, and death.1, 2, 3, 4, 5, 6, 7 Despite these heightened risks, at least 21% of patients are unaware of their AKI diagnosis and kidney-focused follow-up is infrequent.8,9 Appropriate laboratory monitoring with serum creatinine (SCr) or urine protein occurs in just 54% and 14% of patients, respectively, within 6 months of discharge.9,10 Even survivors at the highest risk for poor outcomes, such as those with AKI requiring dialysis, pre-existing CKD, or persistent AKI, are seen by an outpatient nephrologist in only 36-43% of cases.11,12
Increasing attention to post-AKI follow-up through alternative care delivery models may help mitigate these gaps. Nephrologist follow-up is the focal point of most models. Patients involved in AKI survivor clinics directed by nephrologists demonstrate improved kidney health knowledge and adherence to best practices, such as kidney laboratory assessments.8,13 Preliminary data also showed improvements in clinical outcomes, such as blood pressure control and reduced rehospitalization, though confirmatory research is needed.14 This care model has promise, but concerns have been raised about feasibility and scalability. Patients report reluctance to add more doctors to their health care team and cite concerns about travel distance and issues with scheduling follow-up visits.13 Few nephrologists are available in community and rural settings, which decreases access to AKI survivor care.15 Accordingly, nephrologists have called for multidisciplinary care models to enhance capacity for post-AKI care delivery.16
Primary care providers (PCPs) and allied health professionals are well positioned to support the AKI survivor care effort alongside expert consultation from nephrologists.15,17, 18, 19 We therefore developed the AKI in Care Transitions (ACT) program, a multidisciplinary, team-based approach to AKI survivor care based in the primary care setting. This study reports the preliminary feasibility and effectiveness of the ACT program.
Methods
Setting and Participants
This prospective pilot study was conducted between April 2020 and November 2021 at Mayo Clinic in Rochester, Minnesota, a tertiary care center with a primary care practice for local area residents. The Mayo Clinic primary care program includes approximately 150,000 empaneled patients cared for at 7 full-service clinical sites and 2 express care sites in the local counties. Included individuals were adults (≥ 18 years) with Kidney Disease Improving Global Outcomes (KDIGO) stage 3 AKI at any time during their hospitalization who were not discharged on dialysis or with hospice care and who received primary care in a Mayo Clinic Rochester-based clinic.20 Recruitment was limited to patients with stage 3 AKI for feasibility in the pilot stage (Table S1). Excluded patients were non-English speakers, persons cognitively or physically unable to participate (eg, clinician-documented dementia in the electronic health record [EHR]), and persons who did not provide informed consent. Eligibility was determined using an EHR screening alert and EHR review by a study team member. This study was approved by the Institutional Review Board at Mayo Clinic (IRB 20-004204) and registered on ClinicalTrials.gov (NCT04505891).
No formal dedicated AKI survivor clinic exists at Mayo Clinic. There are 5 inpatient nephrology consult services electively available at Mayo Clinic in Rochester, with a typical cumulative daily census of 60-90 patients. Nephrology consult teams include nurse educators whose primary role is to deliver education to hospitalized patients being discharged on dialysis. The primary care practice at Mayo Clinic in Rochester employs a team-based care model that includes physicians, advanced practice providers, nurses, and embedded clinical pharmacists who consult with patients independently or in collaboration with the PCP. There were no significant changes to the standard of transitional post-AKI care within Mayo Clinic or by external consensus during the study period.
In 2020, the previously described ACT program was implemented to provide support for AKI survivors transitioning between the inpatient and outpatient settings and facilitate timely kidney care follow-up after discharge.21 Briefly, AKI survivors identified by the EHR screening alert, an embedded alert that used serum creatinine and urine output data to identify AKI, received inpatient education from nephrology nurse educators approximately 1-3 days before discharge.21 A detailed description of provided education, including artifacts, has been previously published.21 Next, the study team coordinated transitional care, including posthospital visits with a PCP and pharmacist within 14 days after discharge. Nephrology referral was at the discretion of the inpatient nephrologists, if consulted, or the patient’s PCP.
Study Groups
ACT was deployed in 3 phases, which created a natural 3-phase quasi-experimental design, with informed consent obtained for participants in each phase. The first phase (April 2020 to October 2020; ‘Usual Care’ group) included AKI survivors identified by the EHR screening tool who would be candidates for ACT. Patients were passively followed during this phase, and the inpatient care team coordinated any AKI-related education and outpatient follow-up as part of their standard practice. Throughout all phases, any visit could be in-person or virtual, according to patient preference. During the second phase of implementation (October 2020 to April 2021), patients identified by the EHR screening tool were visited by a trained nephrology nurse educator who delivered targeted AKI education using videos, pamphlets, and teach-back strategies before hospital dismissal (the Education Alone group). Frequency and intensity of education were individualized at the discretion of the nephrology nurse educator, but standard components were delivered to all participants. The third and final phase (April 2021 to November 2021) included patients who received the full ACT intervention (the ACT group). In this phase, participants received the previously described nephrology nurse educator AKI education and the study team coordinated outpatient kidney follow-up within 14 days of discharge (Fig 1). Follow-up included discharge orders for laboratory testing (ie, extended metabolic panel including SCr and urinalysis with microscopy or an alternative urine protein test as available) and posthospital follow-up visits with a PCP and a pharmacist, which ideally occurred back-to-back or on the same day. Pharmacists evaluated postdischarge urine protein results and used an established protocol to order a repeat assessment within 3 months if evidence of proteinuria. They also performed a detailed medication review and reconciliation and discussed recommendations with the provider in-person, if possible, or via secure message. Recommendations were at the pharmacists’ discretion and were not standardized or limited to kidney-related medications. Pharmacist recommendations in this workflow are frequently related to therapy optimization (eg, drug choice, dose change), monitoring (eg, drug levels), management of drug interactions, and optimization of patient centeredness (eg, decreased medication burden to improve adherence). The PCP reviewed the posthospital laboratory data, if available, and was encouraged to use the KAMPS (kidney function assessment, awareness and education, medication review, blood pressure monitoring and sick day education) framework (Table S2) for secondary and tertiary prevention of AKI.22 If deemed appropriate, additional follow-up with nephrology or other specialists occurred. Clinical decision support tools were developed and embedded in the EHR during this phase.21 Two alerts developed for inpatient teams included 1) a passive notification that the patient had stage 3 AKI and links to kidney health resources and 2) a failsafe alert to prompt placement of dismissal orders (kidney laboratory monitoring and a PCP and pharmacist visits), if those placed by the ACT study team were discontinued. Clinical decision support was also available for outpatient providers with descriptions of the KAMPS framework and links to additional kidney care resources through a proprietary medical knowledge system.23 Comparisons were made across phases to examine the impact of each added level of intervention on care processes and outcomes.
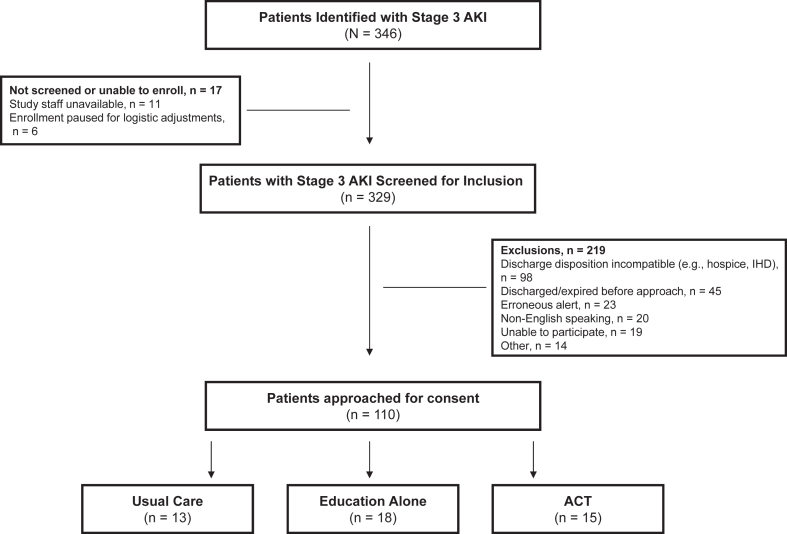
Figure 1.
ACT program implementation phases. During the first phase (‘Usual Care’), participants were identified by the electronic screening tool and passively followed, while the inpatient care team coordinated any education and outpatient follow-up as part of standard practice. In the second phase (‘Education Alone’), standardized kidney health education was delivered to patients and caregivers before hospital dismissal. The third phase (‘ACT’) included standardized education and care coordination of kidney function laboratory tests and provider assessment with 14 days of discharge. During all phases, nephrology follow-up was coordinated at the discretion of the inpatient care team, consulting nephrologists, or primary care provider.
Data Collection
Data abstracted from the EHR included demographics and select comorbid conditions documented in clinician notes. Encounter data included length of hospital and intensive care unit stay, nephrology consultation during hospitalization, and details about the AKI episode. Estimated glomerular filtration rate (eGFR) was determined using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.24 Preadmission SCr was defined as the median SCr from 6 months to 7 days before admission or back-calculated using MDRD (Modification of Diet in Renal Disease) Study equation, assuming an eGFR of 75 mL/min/1.73 m2.25 All data were manually collected from the EHR except laboratory data, which were electronically obtained.
Outcomes and Analysis
Feasibility was measured using the proportion of patients screened, approached for consent, and enrolled from among all patients identified by the EHR alert and the proportion of participants in the ACT group who completed follow-up care (intention-to-treat). Intervention fidelity was measured using the proportion of patients who received the intervention components. We also evaluated the proportion of participants in the ACT group where clinicians interfaced with clinical decision support alerts. Process outcomes assessed in all groups included the frequency and nature of participants’ completed follow-up care, including timing and provider type. The cumulative incidence of provider (PCP or nephrologist) and laboratory (SCr and urine study, including urinalysis with microscopy, urine dipstick, and urine albumin-to-creatinine ratio) follow-up was determined at 14 and 30 days. Clinical outcomes of interest were emergency department visits, hospital readmissions, and death within 90 days. Changes in eGFR between dismissal and 30 ± 15 days and 90 ± 30 days after hospitalization were calculated using outpatient SCr values. Medication data were collected from the discharge summary for the index hospitalization and at 90 days using the medication list from the nearest inpatient or outpatient encounter. Participants were followed for 90 days after hospital dismissal or until death or loss to follow-up within that timeframe.
Continuous data were described using median and interquartile range (IQR). Baseline characteristics, hospitalization, and feasibility data were reported using descriptive statistics. The 3 groups were compared using the Fisher-Freeman-Halton exact test for nominal or discrete data and the Kruskal-Wallis test for continuous data. A sensitivity analysis excluded participants discharged to a skilled nursing facility, as follow-up practices may be impacted by the discharge disposition. As care coordination was facilitated by the study team in only the ACT group, an additional sensitivity analysis compared the ACT group to a group that combined the Usual Care and Education Alone participants. All analyses were performed using SAS version 9.4 software (SAS Institute, Inc.; Cary, NC).
Results
Recruitment Feasibility
Most (329 out of 346) patients identified by the EHR alert were evaluated for inclusion by the study team. There were 219 (67%) exclusions based on prespecified study criteria (Fig 2). Of 110 eligible adults approached, 46 (42%) consented and were enrolled in the study. Participants were primarily White and 41% were women (Table 1). Most participants were admitted for a medical, rather than surgical, indication. The proportion requiring intensive care unit (ICU) monitoring, dialysis during hospitalization, inpatient and nephrology consultation and discharged to a skilled nursing facility was highest in the Education Alone group. All participants had 90-day follow-up data except one in the ACT group, who died within 90 days of hospital dismissal.
Figure 2.
Participant flowchart.
Table 1.
Baseline Characteristic and Hospitalization Data
| Usual Care N=13 |
Education Alone N=18 |
ACT N=15 |
|
|---|---|---|---|
| Baseline Characteristics | |||
| Age at hospitalization, y | 69 (65, 71) | 65 (62, 74) | 68 (57, 78) |
| Female sex | 7 (54) | 10 (56) | 2 (13) |
| White race | 13 (100) | 18 (100) | 14 (93) |
| Comorbid conditions | |||
| Chronic kidney disease | 6 (46) | 11 (61) | 6 (40) |
| Hypertension | 9 (69) | 10 (56) | 14 (93) |
| Cardiovascular disease | 6 (46) | 8 (44) | 10 (67) |
| Diabetes | 7 (54) | 12 (67) | 12 (80) |
| Vascular disease | 4 (31) | 5 (28) | 1 (7) |
| Preadmission SCr, mg/dLa | 1.1 (1.0, 1.4) | 1.2 (0.8, 1.9) | 1.3 (1.2, 2.0) |
| Preadmission eGFR, mL/min/1.73 m2 | 73 (39, 79) | 63 (37, 80) | 58 (34, 71) |
| Hospitalization Data | |||
| Medical admission | 6 (46) | 12 (67) | 14 (93) |
| Cardiovascular | 0 (0) | 3 (25) | 2 (14) |
| Respiratory | 0 (0) | 0 (0) | 1 (7.5) |
| Gastrointestinal | 1 (17) | 1 (8.5) | 3 (21) |
| Genitourinary | 5 (83) | 7 (58) | 7 (50) |
| Other | 0 (0) | 1 (8.5) | 1 (7.5) |
| Surgical admission | 7 (54) | 6 (33) | 1 (7) |
| Cardiothoracic | 2 (29) | 3 (50) | 0 (0) |
| Gastrointestinal | 4 (57) | 2 (33.5) | 1 (100) |
| Other | 1 (14) | 1 (16.5) | 0 (0) |
| ICU admission | 4 (31) | 12 (67) | 5 (33) |
| ICU length of stay, d | 2.5 (2, 3) | 3 (1, 5) | 4 (3, 4) |
| Cause of AKI | |||
| Hypovolemia | 4 (31) | 3 (17) | 3 (20) |
| Sepsis-associated | 1 (8) | 2 (11) | 1 (7) |
| Cardio-renal | 2 (15) | 2 (11) | 3 (20) |
| Obstructive | 1 (8) | 2 (11) | 0 (0) |
| Nephrotoxic | 0 (0) | 0 (0) | 2 (13) |
| Multifactorial | 2 (15) | 6 (33) | 4 (27) |
| Other/Unknown | 3 (23) | 3 (17) | 2 (13) |
| Nephrology consult during hospitalization | 5 (39) | 12 (67) | 8 (53) |
| Dialysis during hospitalization | 0 (0) | 4 (22) | 2 (13) |
| Dismissal SCr, mg/dL | 1.8 (1.3, 2.9) | 2.2 (1.5, 2.8) | 2.2 (1.4, 3.2) |
| Dismissal eGFR, mL/min/1.73 m2 | 40 (16, 58) | 29 (19, 54) | 31 (16, 58) |
| Hospital length of stay, d | 8 (6, 11) | 12 (8, 18) | 9 (5, 12) |
| Discharging service | |||
| Internal/Family medicine | 5 (39) | 9 (50) | 8 (53) |
| Cardiology | 0 (0) | 2 (11) | 3 (20) |
| Oncology/Hematology | 0 (0) | 1 (6) | 0 (0) |
| Medicine, other | 0 (0) | 0 (0) | 2 (13) |
| Cardiovascular surgery | 3 (23) | 3 (17) | 1 (7) |
| Surgical, other | 5 (39) | 3 (17) | 1 (7) |
| Total number of medications at dismissal | 12 (11, 15) | 16 (13, 22) | 16 (8, 21) |
| Participants with medication changes at dismissal | 13 (100) | 18 (100) | 15 (100) |
| Participants with nephrotoxicb medication changes | 6 (46) | 14 (78) | 12 (80) |
| Discharge disposition | |||
| Home | 13 (100) | 10 (56) | 15 (100) |
| Skilled nursing facility | 0 (0) | 5 (28) | 0 (0) |
| Other | 0 (0) | 3 (17) | 0 (0) |
Note: Data reported as n (%) for nominal/discrete data or median (IQR) for continuous data.
Abbreviations: ACT, AKI in Care Transitions; eGFR: estimated glomerular filtration rate; ICU, intensive care unit; SCr, serum creatinine.
Back-calculated using MDRD (Modification of Diet in Renal Disease) Study equation in 2 (Usual Care), 3 (Education Alone), and 1 (ACT) participants.
Nephrotoxic medications included acyclovir, cyclosporin, fluroquinolones, lithium, loop diuretics, methotrexate, nonsteroidal anti-inflammatory drugs, tacrolimus, and sulfamethoxazole-trimethoprim.
Intervention Feasibility
Education was completed by all participants in the Education Alone group and 14 of 15 in the ACT group. Orders for posthospital laboratory monitoring and PCP and pharmacist visits were placed by the study team for all participants in the ACT group except the one patient who did not receive inpatient education. Table 2 outlines the frequency of each intervention element completed in the ACT group. Components delivered outside the target 14-day timeframe included 2 SCr measurements, 1 urine study, and 2 pharmacist visits. Clinicians interfaced with clinical decision support tools for 6 of the 15 ACT patients (40%).
Table 2.
Participants’ Completion of ACT Program Components in the Intention-to-Treat ACT Group During 90-Day Follow-Up
| ACT Program Component | N=15 |
|---|---|
| Inpatient education | 14 (93) |
| Outpatient laboratory monitoring | |
| Serum creatinine | 15 (100) |
| Urine studies | 14 (93) |
| PCP visit | 14 (93) |
| Pharmacist visit | 11 (73) |
Note: Data reported as n (%).
Abbreviations: ACT, AKI in Care Transition; PCP, primary care provider.
Outcomes
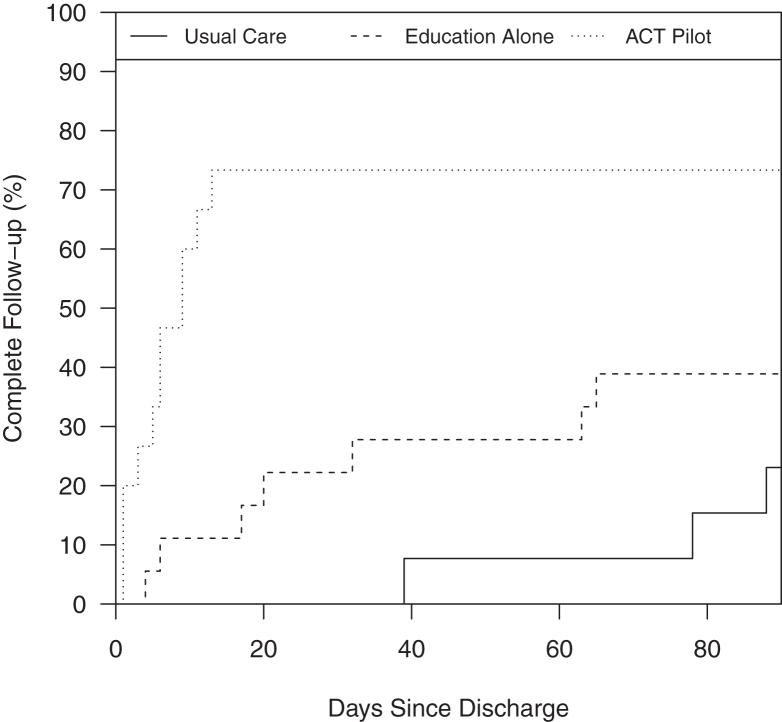
The 14-day cumulative incidence of provider (PCP or nephrologist) and laboratory (SCr and urine study) follow-up was 0% in the Usual Care group, 11% in the Education Alone group, and 80% in the ACT group (P < 0.001; Table 3). The degree of provider and laboratory follow-up was persistently different at 30 days [0%, 22%, and 80%, respectively (P < 0.001)]. Time to follow-up is shown in Figure 3. Findings were consistent in sensitivity analyses excluding those who were discharged to a skilled nursing facility (Table S3) and when the ACT group was compared to the combined Usual Care and Education Alone groups (Table S4).
Table 3.
Kidney Follow-Up Components
| Component | Usual Care N=13 |
Education Alone N=18 |
ACT N=15 |
P Value |
|---|---|---|---|---|
| 14-d provider and laboratory follow-upa | 0 (0) | 2 (11) | 12 (80) | <0.001 |
| 30-d provider and laboratory follow-upa | 0 (0) | 4 (22) | 12 (80) | <0.001 |
| Laboratory monitoring | ||||
| Serum creatinine | ||||
| 14-d | 9 (69) | 11 (61) | 13 (87) | 0.27 |
| 30-d | 11 (85) | 14 (78) | 14 (93) | 0.52 |
| 90-d | 12 (92) | 17 (94) | 15 (100) | 0.74 |
| Time to first assessment, d | 9 (7, 19) | 7 (4, 16) | 7 (2, 6) | 0.03 |
| Urine protein assessmentb | ||||
| 14-d | 0 (0) | 3 (17) | 13 (87) | <0.001 |
| 30-d | 2 (15) | 5 (28) | 13 (87) | <0.001 |
| 90-d | 5 (39) | 9 (50) | 14 (93) | 0.004 |
| Time to first assessment, d | 35 (28, 78) | 20 (7, 52) | 5.5 (2, 9) | 0.001 |
| Clinician follow-up | ||||
| PCPc | ||||
| 14-d | 11 (85) | 8 (44) | 14 (93) | 0.003 |
| 30-d | 12 (92) | 11 (61) | 14 (93) | 0.03 |
| Time to PCP follow-up, d | 9.5 (4, 12.5) | 4 (2, 16) | 5 (1, 8) | 0.29 |
| Nephrologist | ||||
| 14-d | 0 (0) | 1 (6) | 3 (20) | 0.23 |
| 30-d | 1 (8) | 3 (17) | 5 (33) | 0.22 |
| Time to nephrology follow-up, d | 27 | 20 (6, 21) | 14 (13, 22) | 0.50 |
| Pharmacistd | ||||
| 14-d | 1 (8) | 1 (6) | 9 (60) | <0.001 |
| 30-d | 1 (8) | 1 (6) | 11 (73) | <0.001 |
| Time to pharmacist follow-up, d | 13 | 10 | 7 (4, 13) | 0.53 |
Note: Data reported as n (%) for nominal/discrete data or median (IQR) for continuous data.
Abbreviations: ACT, AKI in Care Transition; PCP, primary care provider.
Cumulative incidence of provider (PCP or nephrologist) and laboratory (SCr and urine study) follow-up.
Includes urinalysis with microscopy, urine dipstick, and urine albumin-to-creatinine ratio. Of the 42 urine evaluations performed across the groups within 90 days, 88% were urinalyses or urine dipsticks. In cases where results revealed an elevated protein osmolality ratio or hematuria on screening evaluation with a urinalysis with microscopy or urine dipstick, a repeat assessment and urine albumin-to-creatinine ratio were recommended within 3 months of discharge.
Telehealth visit occurred in 1 and 4 participants in the Usual Care and Education Alone groups, respectively.
Telehealth visit occurred in 1 participant in the ACT group.
Figure 3.
Time to provider (PCP or nephrologist) and laboratory (SCr and urine study) follow-up across 3 groups. A significantly greater proportion of patients achieved provider and laboratory follow-up in the ACT group (P < 0.001).
Thirty-day readmission rates were 23%, 44%, and 20% in the Usual Care, Education Alone, and ACT groups, respectively (P = 0.25; Table 4). The median number of days (interquartile range [IQR]) to readmission was 29 (25, 36), 13 (7, 57), and 49 (8, 77) in the Usual Care, Education Alone, and ACT groups, respectively (P = 0.54). There was no significant difference in eGFR changes between the groups at 30 or 90 days (Table 4).
Table 4.
Clinical Outcomes
| Usual Care N=13 |
Education Alone N=18 |
ACT N=15 |
P Value | |
|---|---|---|---|---|
| 30-d readmission | 3 (23) | 8 (44) | 3 (20) | 0.25 |
| 90-d readmission | 5 (39) | 11 (61) | 6 (40) | 0.35 |
| Days to 1st readmission | 29 (25, 36) | 13 (7, 57) | 49 (8, 77) | 0.67 |
| Kidney-related readmission | 0.69 | |||
| Definite | 2 (40) | 3 (27) | 1 (17) | |
| Possible | 0 | 2 (18) | 2 (33) | |
| Doubtful | 3 (60) | 6 (55) | 3 (50) | |
| 90-d ED visit | 5 (39) | 6 (33) | 4 (27) | 0.86 |
| Days to 1st ED visit | 18 (4, 73) | 55 (27, 63) | 6 (2, 37) | 0.33 |
| 30-d ED visit or readmission | 3 (23) | 9 (50) | 5 (33) | 0.29 |
| 90-d ED visit or readmission | 6 (46) | 14 (78) | 8 (53) | 0.16 |
| Days to 1st ED visit or readmission | 20 (2, 73) | 15 (7, 63) | 16 (4, 769) | 0.84 |
| 90-d mortality | 0 (0) | 0 (0) | 1 (7) | 0.35 |
| Change in eGFRa from dismissal to day 30 | 6 (-14, 10) | 7 (2, 11) | 6 (-3, 21) | 0.84 |
| Change in eGFRa from dismissal to day 90 | 4 (-2, 15) | 7 (3, 26) | 3 (-7, 14) | 0.50 |
Note: Data reported as n (%) for nominal/discrete data or median (IQR) for continuous data.
Abbreviations: ACT, AKI in Care Transition; ED, emergency department; eGFR, estimated glomerular filtration rate.
mL/min/1.73 m2
Participants were on a median (IQR) of 12 (11, 15) medications in the Usual Care group at hospital discharge, 16 (13, 22) in the Education Alone group, and 16 (8, 21) in the ACT group (Table 1). Two (15%) participants in the Usual Care group had a nonsteroidal anti-inflammatory drug on their medication list at hospital discharge compared to zero in the Education Alone and ACT groups (P = 0.07; Table 5). Renoprotective medications, including renin-angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors, were newly initiated within 90 days in 3 (20%) ACT participants compared to 0 and 1 (6%) in the Usual Care and Education Alone groups, respectively (P = 0.23; Table 5).
Table 5.
Patterns of Medication Use
| Drug/Class | Hospital Discharge |
90 d |
||||||
|---|---|---|---|---|---|---|---|---|
| Usual Care N=13 |
Education Alone N=18 |
ACT N=15 |
P-Value | Usual Care N=13 |
Education Alone N=18 |
ACT N=15 |
P Value | |
| ACEi | 2 (15) | 3 (17) | 2 (13) | 0.97 | 2 (15) | 4 (22) | 4 (27) | 0.77 |
| ARB | 2 (15) | 0 (0) | 1 (7) | 0.23 | 2 (15) | 0 (0) | 0 (0) | 0.07 |
| NSAID | 2 (15) | 0 (0) | 0 (0) | 0.07 | 1 (8) | 0 (0) | 0 (0) | 0.27 |
| Loop diuretic | 5 (39) | 9 (50) | 4 (27) | 0.39 | 5 (39) | 9 (50) | 2 (13) | 0.08 |
| Spironolactone | 0 (0) | 0 (0) | 1 (7) | 0.35 | 1 (8) | 0 (0) | 1 (7) | 0.51 |
| SGLT2i | 0 (0) | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | 1 (7) | 0.35 |
| SMX/TMP | 1 (8) | 1 (6) | 2 (13) | 0.72 | 1 (8) | 0 (0) | 1 (7) | 0.51 |
| Calcineurin inhibitor | 1 (8) | 3 (17) | 1 (7) | 0.60 | 1 (8) | 3 (17) | 0 (0) | 0.24 |
Note: Data reported as n (%).
Abbreviations: ACT, AKI in Care Transition; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; NSAID, nonsteroidal anti-inflammatory drug; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SMX/TMP, sulfamethoxazole-trimethoprim.
Discussion
In this prospective evaluation of a multidisciplinary model for AKI survivor care, we demonstrated that kidney health education and coordinated follow-up of AKI survivors in primary care increases timely adherence to best practices. Feasibility was evidenced by effective participant identification and recruitment practices, which yielded a 42% enrollment rate, higher than reported with nephrologist-centric programs, and reliable delivery of the ACT intervention, with >80% of participants completing education and postdischarge laboratory and provider follow-up.
In this study, participation in the ACT program was associated with a higher rate of laboratory monitoring for kidney function assessment and provider follow-up, 2 core components of best practices for high-quality post-AKI care.22 It appeared that improved completion of timely urine protein evaluation was a key driver in the ACT program impact. Although urine protein assessment is a key prognostic indicator in AKI survivors, United States Renal Data System data indicate that it was evaluated in less than 20% of patients in the 6 months after discharge.26,27 A mixed methods study from the ACT group indicated that this is likely due to a combination of factors, including a lack of awareness, competing priorities, and opportunities to improve kidney knowledge and education among PCPs.28 An episode of AKI has been associated with a 9% increase in urine albumin-to-creatinine ratio, with greater increases following more severe AKI (eg, 24% with stage 3 AKI).29 Identifying and quantifying proteinuria is a critical risk-stratification tool following AKI. A higher urine albumin-to-creatinine ratio is associated with an increased risk of kidney disease progression and dialysis needs.26,30 It also has implications beyond kidney disease, such as complications from cardiovascular disease, and thus may inform other comorbid condition-directed therapy.31 When used in the primary care setting, identification of proteinuria may identify a subset of patients where nephrology consultation would provide greatest benefit. The higher rates of urine protein assessment observed in the ACT group were likely driven primarily by the active role of the ACT study team in care coordination of clinical and laboratory follow-up before hospital dismissal. Urine protein test selection was driven by availability at the primary care practice site. While the urine albumin-to-creatinine ratio would be preferred for all patients, semiquantitative assessments with a urine dipstick or urinalysis with microscopy may be more feasible in certain environments. For patients with evidence of proteinuria on surveillance evaluation, a more detailed review is warranted. An established pharmacist-driven, collaborative practice protocol facilitated ordering repeat measurements for participants in the ACT group with elevated protein on initial assessment. Overall, monitoring for proteinuria may catalyze the initiation or resumption of renoprotective medications during postdischarge follow-up, as was seen in 20% of the ACT group compared to 0% and 6% in the Usual Care and Education Alone groups, respectively.
ACT participants had a significantly decreased time to posthospital laboratory monitoring and PCP and pharmacist visits. The frequency of nephrologist visits and the time to nephrologist follow-up was similar across groups. This represents an improvement relative to previously described nephrologist-centric models, which showed a median follow-up time of 15-48 days.13,14 Early posthospital follow-up may allow for more timely recognition of AKI-related complications, decreased exposure to nephrotoxins, and adjustment of diuretic and other medication doses during the dynamic arc of kidney recovery. A prior report from this study identified a median of 3 drug therapy problems per patient, 18% of which were for nephrotoxic/renoprotective medication optimization. Among these pharmacist-identified interventions for renally active therapy, 80% of optimization recommendations were acted on by providers within 7 days.32 Previous research on collaborative delivery of post-AKI care between nephrologists and PCPs found that follow-up in primary care, in advance of or in conjunction with nephrologist follow-up when indicated, was seen as instrumental in assuring care continuity and comorbidity management.28 Similar models of transitional, multidisciplinary team-based follow-up in primary care have demonstrated reductions in ED visits, rehospitalization, and costs as well as improved self-rated health.33, 34, 35, 36 Collaborative care delivery has been identified as desirable and necessary for the scale and spread of post-AKI care, and further research on how it may be optimized is warranted.16,37, 38, 39, 40
By incorporating multiple disciplines, including pharmacists and nephrology nurse educators, this health care delivery model capitalizes on specialty knowledge while reducing burden on already limited provider resources. Multidisciplinary engagement, particularly pharmacist-led medication review and reconciliation, has been recommended as a foundational element of post-AKI care by nephrologists and patients.16,37, 38, 39, 40 Medication management is one of the few modifiable determinants of patient outcomes, and pharmacist involvement in transitions of care has been associated with reduced hospital readmissions and polypharmacy.41, 42, 43, 44, 45 Despite these factors, their routine incorporation into post-AKI care is not well documented. In the present study, pharmacist involvement may have contributed to favorable medication use patterns, including lack of nonsteroidal anti-inflammatory drugs and initiation of renoprotective medications in 20% of participants the ACT group.32
This model of care included several strengths that address known barriers to delivery of post-AKI care. Use of an EHR-based screening tool allowed for automated identification of AKI survivors from among the entire inpatient census. Previous tactics have relied on time-consuming manual efforts, including review of cases in select hospital locations (eg, an ICU) and/or referral from an inpatient nephrology consult team.13,14,46 Such approaches may miss AKI survivors who stand to benefit significantly from kidney follow-up care. As a representative example, among those included in our study, only 54% were seen by nephrology during their hospitalization, and 46% had an ICU stay. Engagement of primary care may have contributed to a higher participation rate than observed in other nephrology-centric models.13 Patients have described reluctance to add additional specialists to their care team and long wait times for access to specialists as barriers to participating in post-AKI care.13,47 Targeted AKI education before hospital dismissal may have increased patient awareness about AKI and knowledge about the importance of kidney health follow-up, which are additional obstacles to patient participation in follow-up.8,40,48 This may have contributed to high compliance with ACT program components, including laboratory monitoring (93-100%) and provider visits (80%). Clinical decision support tools may act as important prompts to coordinate recommended follow-up care and thus contributed to the success of the ACT program. However, as only 40% of providers interfaced with these tools, more research is needed to optimize their utility. Collectively, this study provides evidence for the potential scalability and generalizability of this approach to post-AKI health care delivery.
This study is not without limitations. Patients were recruited during phased implementation of the ACT program and were not randomly assigned to treatment groups, which likely contributed to differences across groups. As the primary outcome was feasibility, the sample size was small and thus insufficient to detect differences in many clinically meaningful outcomes or perform multivariable analysis. Given these factors, findings related to clinical outcomes should be interpreted with caution. Dismissal to a skilled nursing facility occurred at varying rates across groups and may have impacted the timing and frequency of postdischarge follow-up. Thus, a sensitivity analysis was performed excluding patients who dismissed to a skilled nursing facility and results were similar. There also remains a need to evaluate key patient-reported outcomes, including the effect of ACT on kidney health knowledge, which is planned but beyond the scope of this report. All participants were receiving primary care in the same region as the tertiary care center where recruitment was conducted, and all sites use a shared EHR. This minimizes the likelihood of missing follow-up or rehospitalization outside our health system and may affect generalizability of these data. It is unknown how our findings translate to patients receiving primary care at a greater distance or in practices that do not share an EHR with the discharging hospital. Additionally, this study used a proactive approach to post-AKI care coordination, with the study team facilitating recruitment and delivery of the education and arranging clinical and laboratory follow-up before hospital dismissal. Large-scale feasibility cannot be inferred from these data. Additional personnel, adaptive workflows, or automation may be necessary to facilitate scale and spread. A dedicated nurse navigator or care manager for AKI survivors would likely be of great benefit to extending the reach of programs, such as ACT, to more patients.14 Nevertheless, a primary care-based follow-up strategy is likely more feasible in these circumstances than a nephrologist-driven specialty clinic, as has been previously reported for AKI survivors. Finally, the gap between the number of patients screened (n=329) and approached for consent (n=110) is evidence of the heterogeneity of the AKI survivor population, challenges with electronic identification of AKI survivor candidates, and complexity of care delivery. Although primary care-based follow-up of AKI survivors may offer significant benefits in select populations, a one-size-fits-all approach is unlikely to be successful. Follow-up pathways should be flexible to accommodate diversity in patients and clinical scenarios.
In conclusion, this pilot study demonstrated feasibility of multidisciplinary AKI survivor follow-up beginning in primary care, with higher 14- and 30-day cumulative incidence of laboratory and provider follow-up in ACT participants. Further studies are needed to determine the effect on important clinical and patient-centered outcomes and to identify strategies for optimizing collaborative care delivery between nephrologists and the primary care team.
Article Information
ACT Study Group
The ACT Study Group comprises the authors listed below.
Authors’ Full Names and Academic Degrees
Heather P. May, PharmD, MSc, Joseph R. Herges, PharmD, Brenda K. Anderson, RN, Gregory J. Hanson, MD, Kianoush B. Kashani, MD, Andrea G. Kattah, MD, Kristin C. Cole, MS, Rozalina G. McCoy, MD, MS, Laurie A. Meade, RN, Andrew D. Rule, MD, Diana J. Schreier, PharmD, MBA, Angeliki G. Tinaglia, RRT, LRT, and Erin F. Barreto, PharmD, MSc on behalf of the ACT Study Group.
Authors’ Contributions
Study design: HPM, EFB, JRH, DJS, KBK, AGK, RGM, ADR, and GJH; data interpretation: HPM, EFB, JRH, DJS, KBK, AGK, RGM, ADR, and GJH; study enrollment BKA, LAM, AGT; data collection and reporting: BKA, LAM, AGT; statistical analysis: KCC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported in part by the Mayo Midwest Pharmacy Research Committee, Mayo Midwest Clinical Practice Committee Innovation Award, American College of Clinical Pharmacy, the National Institute of Allergy and Infectious Diseases of under award number K23AI143882 (PI; EFB), and the Agency for Healthcare Research and Quality HS028060-01 (PI; EFB). The funding sources had no role in study design; data collection, analysis, or interpretation; writing the report; or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We would like to acknowledge the valuable contributions of Shelley Preble, Kate Mayhew, and Sophea Seng to this project.
Data Sharing
Source data may be made available upon reasonable request.
Peer Review
Received March 24, 2023. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 10, 2023.
Footnotes
Complete author and article information provided before references.
Table S1: KDIGO criteria for acute kidney injury.
Table S2: KAMPS framework for components of kidney follow-up care.
Table S3: Analysis of kidney follow-up components excluding participants discharged to a skilled nursing facility.
Table S4: Analysis of kidney follow-up components by team responsible for postdischarge care coordination.
Supplementary Material
Tables S1-S4.
References
- 1.Kashani K., Shao M., Li G., et al. No increase in the incidence of acute kidney injury in a population-based annual temporal trends epidemiology study. Kidney Int. 2017;92(3):721–728. doi: 10.1016/j.kint.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siew E.D., Parr S.K., Abdel-Kader K., et al. Predictors of recurrent AKI. J Am Soc Nephrol. 2016;27(4):1190–1200. doi: 10.1681/ASN.2014121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannu N., James M., Hemmelgarn B., et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heung M., Steffick D.E., Zivin K., et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis. 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawhney S., Marks A., Fluck N., et al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92(2):440–452. doi: 10.1016/j.kint.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odutayo A., Wong C.X., Farkouh M., et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377–387. doi: 10.1681/ASN.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz-Soriano V., Alcorn J.L., Li X., et al. A survey study of self-rated patients’ knowledge about AKI in a post-discharge AKI clinic. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119830700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saran R., Robinson B., Abbott K.C., et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 Suppl 1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreto E.F., Schreier D.J., May H.P., et al. Incidence of serum creatinine monitoring and outpatient visit follow-up among acute kidney injury survivors after discharge: a population-based cohort study. Am J Nephrol. 2021;52(10-11):817–826. doi: 10.1159/000519375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karsanji D.J., Pannu N., Manns B.J., et al. Disparity between nephrologists’ opinions and contemporary practices for community follow-up after AKI hospitalization. Clin J Am Soc Nephrol. 2017;12(11):1753–1761. doi: 10.2215/CJN.01450217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harel Z., Wald R., Bargman J.M., et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83(5):901–908. doi: 10.1038/ki.2012.451. [DOI] [PubMed] [Google Scholar]
- 13.Silver S., Adhikari N., Bell C., et al. Nephrologist Follow-Up versus Usual Care after an Acute Kidney Injury Hospitalization (FUSION) Clin J Am Soc Nephrol. 2021;16(7):1005–1014. doi: 10.2215/CJN.17331120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh G., Hu Y., Jacobs S., et al. Post-discharge mortality and rehospitalization among participants in a comprehensive acute kidney injury rehabilitation program. Kidney360. 2021;2(9):1424–1433. doi: 10.34067/KID.0003672021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greer R.C., Liu Y., Cavanaugh K., et al. Primary care physicians’ perceived barriers to nephrology referral and co-management of patients with CKD: a qualitative study. J Gen Intern Med. 2019;34(7):1228–1235. doi: 10.1007/s11606-019-04975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver S.A., Siew E.D. Follow-up care in acute kidney injury: lost in transition. Adv Chronic Kidney Dis. 2017;24(4):246–252. doi: 10.1053/j.ackd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Brimble K.S., Boll P., Grill A.K., et al. Impact of the KidneyWise toolkit on chronic kidney disease referral practices in Ontario primary care: a prospective evaluation. BMJ Open. 2020;10(2) doi: 10.1136/bmjopen-2019-032838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neale E.P., Middleton J., Lambert K. Barriers and enablers to detection and management of chronic kidney disease in primary healthcare: a systematic review. BMC Nephrol. 2020;21(1):83. doi: 10.1186/s12882-020-01731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haley W.E., Beckrich A.L., Sayre J., et al. Improving care coordination between nephrology and primary care: a quality improvement initiative using the renal physicians association toolkit. Am J Kidney Dis. 2015;65(1):67–79. doi: 10.1053/j.ajkd.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 20.KDIGO KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl (2011) 2012;2(1):4. [Google Scholar]
- 21.Barreto E.F., May H.P., Schreier D.J., et al. Development and feasibility of a multidisciplinary approach to AKI survivorship in care transitions: research letter. Can J Kidney Health Dis. 2022;9 doi: 10.1177/20543581221081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashani K., Rosner M.H., Haase M., et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14(6):941–953. doi: 10.2215/CJN.01250119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North F., Fox S., Chaudhry R. Clinician time used for decision making: a best case workflow study using cardiovascular risk assessments and Ask Mayo Expert algorithmic care process models. BMC Med Inform Decis Mak. 2016;16:96. doi: 10.1186/s12911-016-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey A.S., Bosch J.P., Lewis J.B., et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hsu C.Y., Chinchilli V.M., Coca S., et al. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the assessment, serial evaluation, and subsequent sequelae in acute kidney injury (ASSESS-AKI) study. JAMA Intern Med. 2020;180(3):402–410. doi: 10.1001/jamainternmed.2019.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen K.L., Chertow G.M., Gilbertson D.T., et al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2022;79(4 Suppl 1):A8–A12. doi: 10.1053/j.ajkd.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May H.P., Krauter A.K., Finnie D.M., et al. Acute kidney injury survivor care following hospital discharge: A mixed-methods study of nephrologists and primary care providers. Kidney Med. 2023;5(4) doi: 10.1016/j.xkme.2022.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu C.Y., Hsu R.K., Liu K.D., et al. Impact of AKI on urinary protein excretion: analysis of two prospective cohorts. J Am Soc Nephrol. 2019;30(7):1271–1281. doi: 10.1681/ASN.2018101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James M.T., Pannu N., Hemmelgarn B.R., et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318(18):1787–1797. doi: 10.1001/jama.2017.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita K., Coresh J., Sang Y., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet Diabetes Endocrinol. 2010;375:514–525. [Google Scholar]
- 32.Herges J.R., May H.P., Meade L.A., et al. Pharmacist-provider collaborative visits after hospital discharge in a comprehensive acute kidney injury survivor model. J Am Pharm Assoc (2003) 2023;63(3):909–914. doi: 10.1016/j.japh.2022.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman E.A., Parry C., Chalmers S., et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 34.Wee S.L., Loke C.K., Liang C., et al. Effectiveness of a national transitional care program in reducing acute care use. J Am Geriatr Soc. 2014;62(4):747–753. doi: 10.1111/jgs.12750. [DOI] [PubMed] [Google Scholar]
- 35.Gardner R., Li Q., Baier R.R., et al. Is implementation of the care transitions intervention associated with cost avoidance after hospital discharge? J Gen Intern Med. 2014;29(6):878–884. doi: 10.1007/s11606-014-2814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi P.Y., Naessens J.M., Peterson S.M., et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthcare. 2016;4(1):30–35. doi: 10.1016/j.hjdsi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Vijayan A., Abdel-Rahman E.M., Liu K.D., et al. Recovery after critical illness and acute kidney injury. Clin J Am Soc Nephrol. 2021;16(10):1601–1609. doi: 10.2215/CJN.19601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K.D., Forni L.G., Heung M., et al. Quality of care for acute kidney disease: current knowledge gaps and future directions. Kidney Int Rep. 2020;5(10):1634–1642. doi: 10.1016/j.ekir.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phipps D.L., Morris R.L., Blakeman T., et al. What is involved in medicines management across care boundaries? A qualitative study of healthcare practitioners’ experiences in the case of acute kidney injury. BMJ Open. 2017;7(1) doi: 10.1136/bmjopen-2016-011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siew E.D., Liu K.D., Bonn J., et al. Improving care for patients after hospitalization with acute kidney injury. J Am Soc Nephrol. 2020;31(10):2237–2241. doi: 10.1681/ASN.2020040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurani S.S., Lampman M.A., Funni S.A., et al. Association between area-level socioeconomic deprivation and diabetes care quality in US primary care practices. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurani S., Hickson L.T.J., Thorsteinsdottir B., et al. Supplement use by US adults with CKD: a population-based study. Am J Kidney Dis. 2019;74(6):862–865. doi: 10.1053/j.ajkd.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreier D.J., Rule A., Kashani K.B., et al. Nephrotoxin exposure in the 3 years following hospital discharge predicts development or worsening of chronic kidney disease among acute kidney injury survivors. Am J Nephrol. 2022;53(4):273–281. doi: 10.1159/000522139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herges J.R., Herges L.B., Dierkhising R.A., et al. Effect of postdismissal pharmacist visits for patients using high-risk medications. Mayo Clin Proc Innov Qual Outcomes. 2018;2(1):4–9. doi: 10.1016/j.mayocpiqo.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal A., Babbott S., Wilkinson S.T. Can the targeted use of a discharge pharmacist significantly decrease 30-day readmissions? Hosp Pharm. 2013;48(5):380–388. doi: 10.1310/hpj4805-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silver S.A., Goldstein S.L., Harel Z., et al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36. doi: 10.1186/s40697-015-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silver S.A., Saragosa M., Adhikari N.K., et al. What insights do patients and caregivers have on acute kidney injury and posthospitalisation care? A single-centre qualitative study from Toronto, Canada. BMJ Open. 2018;8(6) doi: 10.1136/bmjopen-2017-021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siew E.D., Parr S.K., Wild M.G., et al. Kidney disease awareness and knowledge among survivors of acute kidney injury. Am J Nephrol. 2019;49(6):449–459. doi: 10.1159/000499862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4.