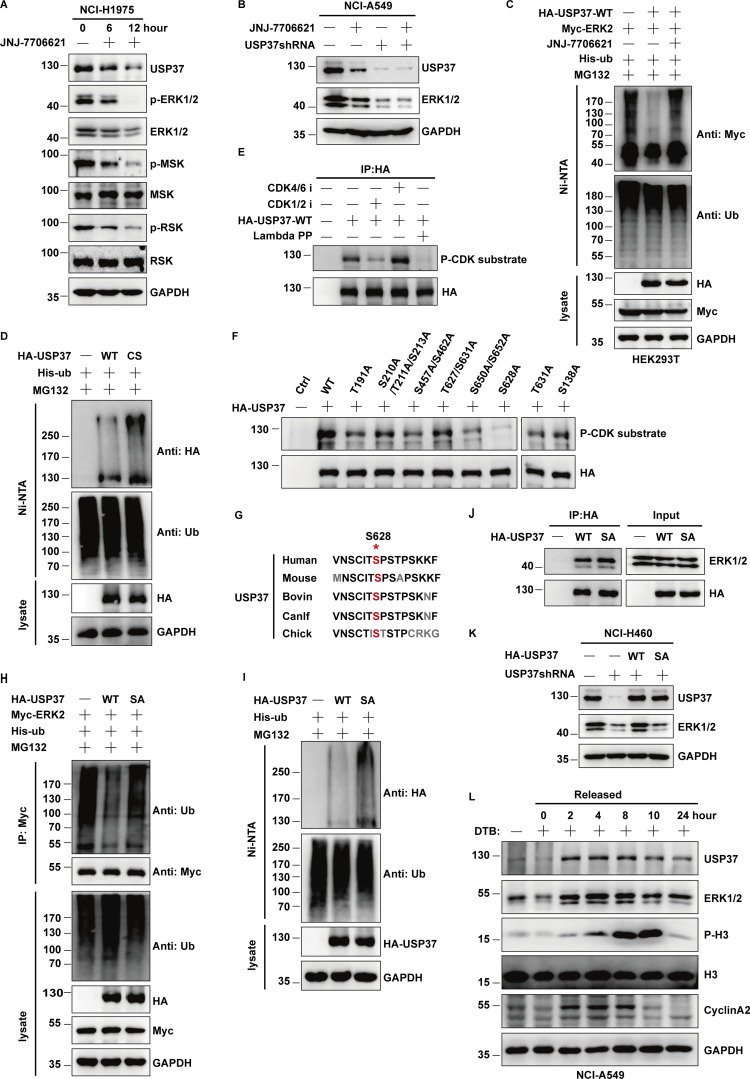
Figure 6.
CDK2 regulates ERK1/2 stability by phosphorylating and activating USP37. (A) Cells were untreated or treated with JNJ-7706621 for three different point times (0, 6, and 12 h) before harvest, and then cell lysates were blotted with the indicated antibodies. (B) Cells stably expressing control shRNA or USP37shRNA were with/without the JNJ-7706621 before harvest, and then cell lysates were blotted with the indicated antibodies. (C) Cells transfected with as indicated constructs were treated with as indicated. Covalently modified proteins purified on NiNTA-agarose under denatured conditions. Ubiquitinated ERK1/2 was detected by anti-Myc antibody. (D) Cells transfected with as indicated plasmids were treated with MG132 for 10 h before being harvested. Covalently modified proteins purified on NiNTA-agarose under denatured conditions. Ubiquitinated USP37 was detected by anti-HA antibody. (E) HA-USP37 WT was transiently transfected into HEK293T cells. These cells were subsequently treated with vehicle, CDK1/2i, or CDK4/6i for 12 h. Cells were lysed and purified using anti–HA-agarose beads. One of the samples was additionally treated with Lambda PPase as indicated. The immunoprecipitates were then blotted with the indicated antibodies. (F) USP37 WT or several USP37 SA mutant plasmids were transfected into HEK293T cells, respectively. Cell lysates were immunoprecipitated with HA beads and immunoblotted as indicated. (G) Sequence alignment of USP37 in various species. *: phosphorylation site. (H) HEK293T cells were transfected with Myc-ERK2 plasmid together with His-ub, and then treated with as indicated. Cell lysates were purified with anti-Myc beads and immunoblotted with indicated antibodies. (I) Cells transfected with the indicated constructs were treated with MG132 for 10 h before being harvested. Covalently modified proteins purified on NiNTA-agarose under denatured conditions. Ubiquitinated USP37 was detected by anti-HA antibody. (J) Cells transfected with the indicated constructs. After transfection 48 h, cells were lysed and purified using anti–HA-agarose beads. The immunoprecipitates were then blotted with the indicated antibodies. (K) Cells stably expressing control shRNA or USP37shRNA were transfected with the indicated constructs were lysed, and cell lysates were blotted with the indicated antibodies. (L) Cells were treated with double thymidine block (DTB) and then released as indicated time points, and then the cell lysates were blotted with the indicated antibodies. Source data are available for this figure: SourceData F6.