Scheme 2.
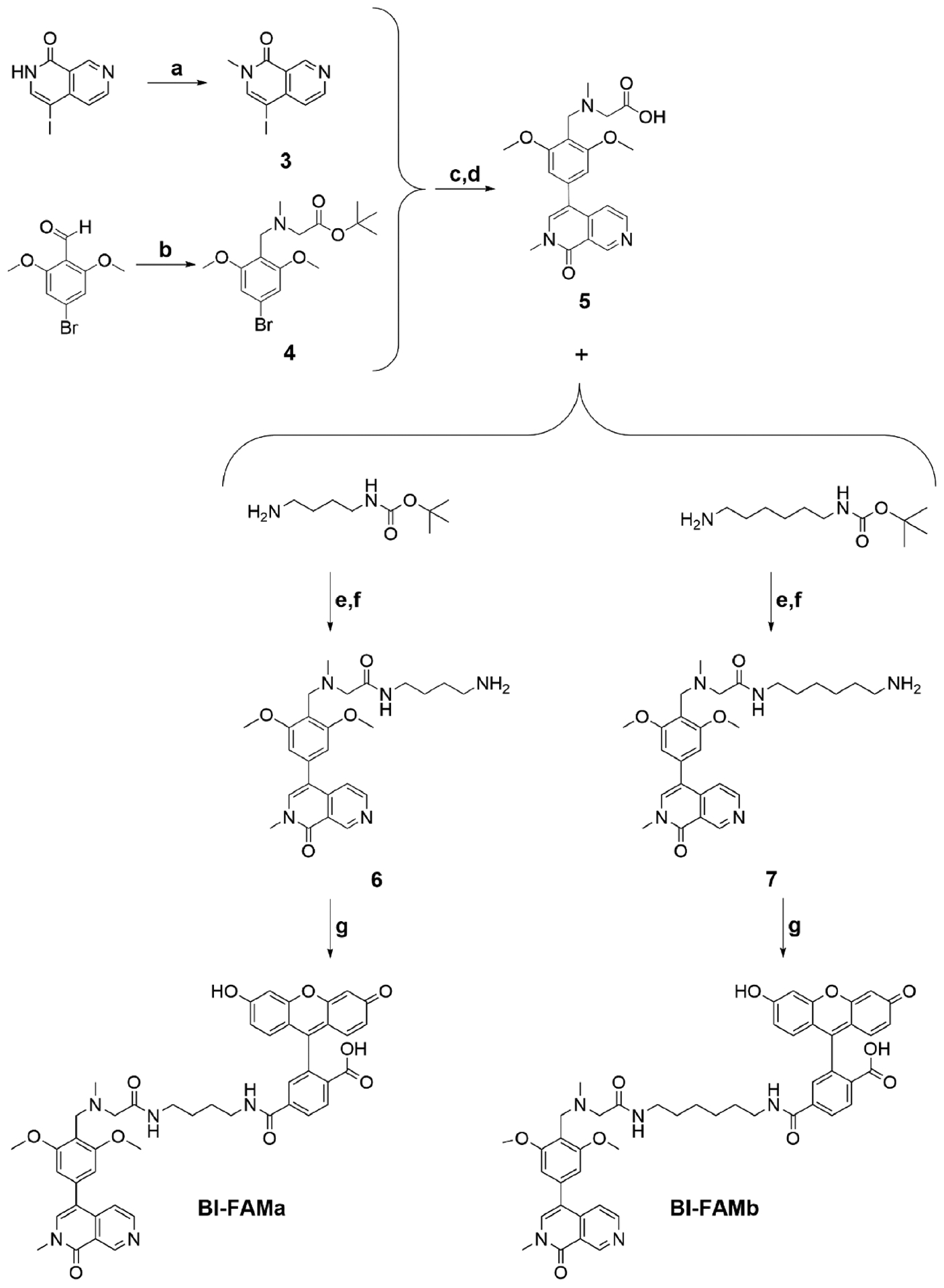
Reagents and Conditions for the Synthesis of BI-FAMa and BI-FAMba
a(a) NaH, DMF (anhydrous), CH3I at 0 °C warm to rt, 16 h; (b) sarcosyl tert-butyl ester hydrochloride, DCM, NaOAc, then acetic acid, NaBH(OAc)3, 18 h, then 1 M K2CO3 to pH = 11; (c) 4, bis(pinacolato)diboron, DMF, KOAc, PdCl2(dppf)CH2Cl2, 16 h, 90 °C, under N2, then 3 and 1 M K2CO3, 80 °C, 16 h; (d) 80% TFA, rt, 2 h; (e) DMF activated with acetic acid/DIC, rt, 12−16 h; (f) 75% TFA, DCM, rt, 16 h; (g) NHS-fluorescein, DMSO/PBS pH = 8.2 1:1, protected from light, rt, 4 h.
