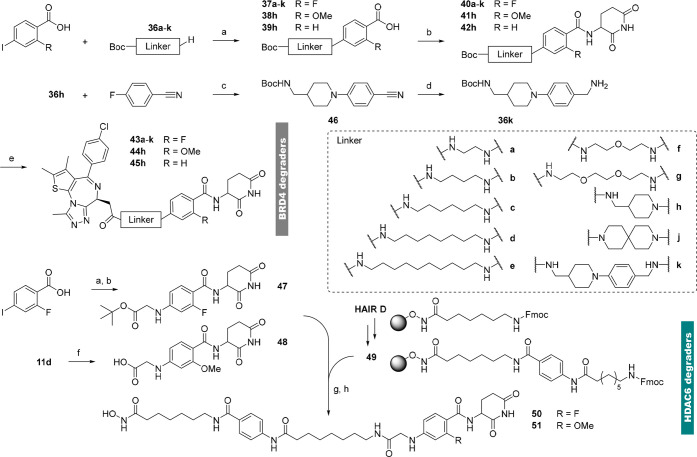
Scheme 4. Synthesis of Benzamide-Type PROTACs Targeting BRD4 or HDAC6.
Reagents and conditions: (a) (i) aryl iodide, CuI, l-proline, DMSO, 5 min; (ii) primary or secondary amines, DMSO, rt, 3 d, 19–79%; (b) EDC × HCl, HOBt, 3-aminopiperidine-2,6-dione hydrochloride, DIPEA, DMF, rt, 16 h, 27–82%; (c) DIPEA, DMSO, 90 °C, 16 h, 51%; (d) CoCl2, NaBH4, MeOH, 0 °C, 2 h, 22%; (e) (i) TFA, CH2Cl2, rt, 2 h; (ii) (+)-JQ1 carboxylic acid, HATU, DIPEA, DMF, rt, 16 h, 31–92%; (f) (i) glyoxylic acid monohydrate, NaOAc, AcOH, MeOH, 0 °C; (ii) NaCNBH3, 0 °C, 1 h, 29%; (g) (i) 47, TFA, CH2Cl2, rt, 2 h or 48; (ii) 49, 20% piperidine, DMF, rt, 2 × 5 min; (iii) 47-COOH or 48-COOH, 49-NH2, HATU/EDC × HCl, HOBt × H2O, DIPEA, DMF, rt, 18 h; (h) TFA, triisopropylsilane, CH2Cl2, rt, 1 h, 28–33% (7 steps).