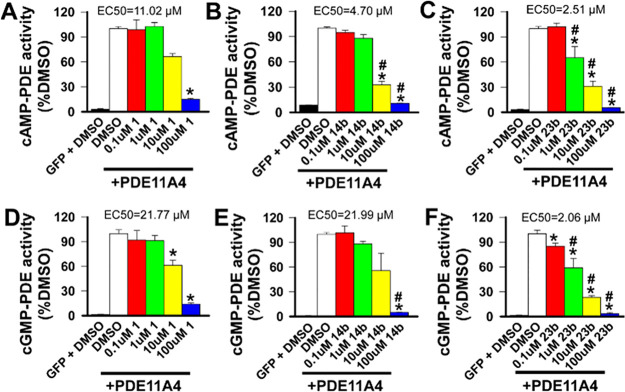
Figure 2.
Efficacy of PDE11A inhibitors in a cell model of aging-like PDE11A4 protein abnormalities. (A) 1, (B) 14b, and (C) 23b all reduce PDE11A4 cAMP hydrolytic activity, but 23b appears more potent as it was the only compound with robust inhibition noted at 1 μM (note that 1 and 23b were simultaneously cultured and processed). The same pattern was observed for the ability of (D) 1, (E) 14b, and (F) 23b to inhibit PDE11A4 cGMP hydrolytic activity, again with 23b exhibiting greater potency. Comparison of 100 μM dose groups across compounds suggests that 14b and 23b are both more efficacious than 1, with both showing stronger inhibition of PDE11A4 cAMP and cGMP hydrolytic activity. *vs DMSO + PDE11A4 within experiment, P < 0.05–0.001; #vs 1 at the same concentration, P < 0.05–0.001. Data graphed mean ± SEM.