Abstract
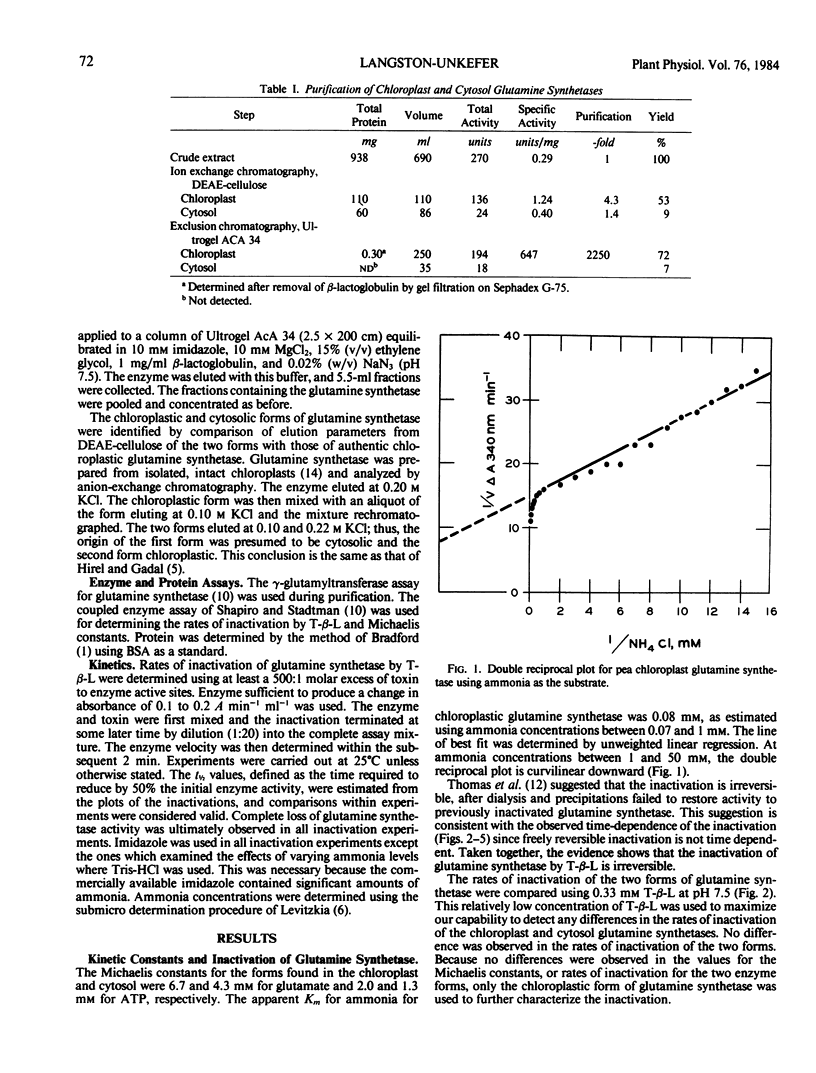
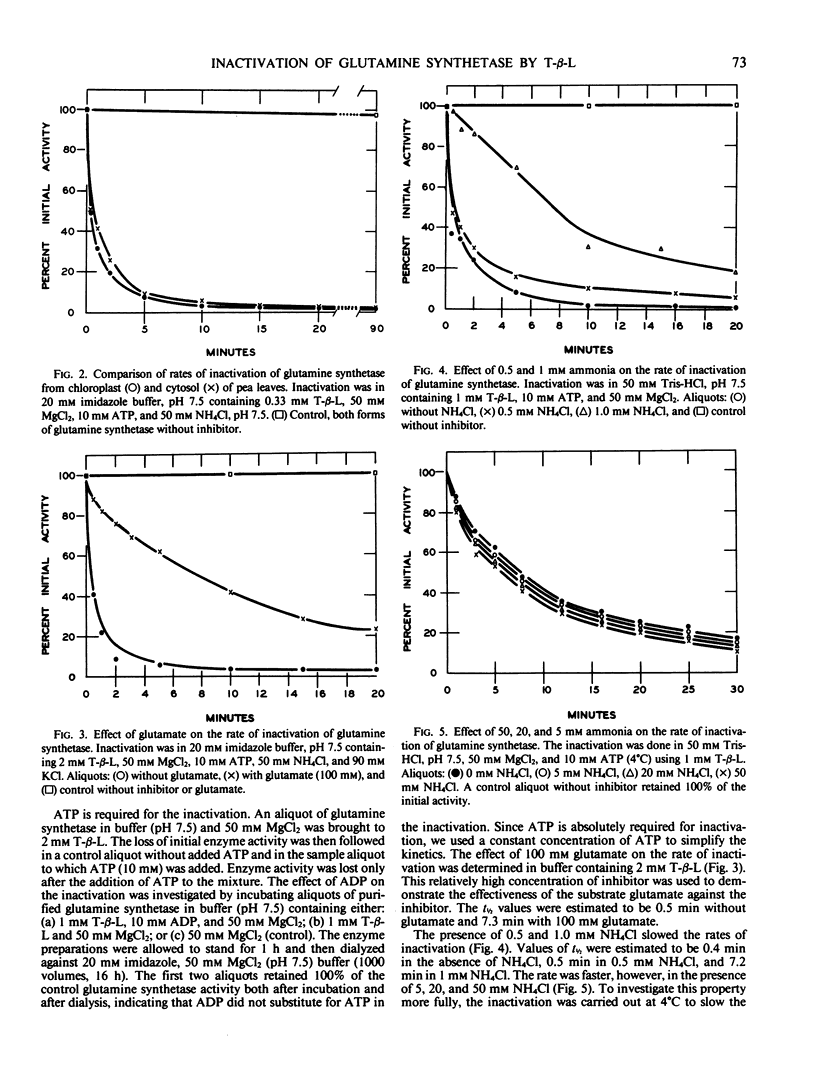
The inactivation of glutamine synthetase by tabtoxinine-β-lactam, a phytotoxin produced by Pseudomonas syringae pv. tabaci, was shown to be irreversible. The chloroplast and cytosolic forms of the enzyme from pea leaves (Pisum sativum L.) were separated, purified, and found to be kinetically similar with Km values for glutamate of 6.7 and 4.3 millimolar and for ATP of 2.0 and 1.3 millimolar, respectively. Both forms were irreversibly inactivated by the toxin at equal rates. Using the chloroplast form, it was found that inactivation by tabtoxinine-β-lactam required ATP. Glutamate and low levels of ammonia (<2 millimolar) slowed the rate of inactivation, whereas high levels of ammonia (5, 20, and 50 millimolar) accelerated it. The inactivation proceeded at a faster rate as the pH was increased from pH 6.5 to 7.5. The role which cellular compartmentalization could play in the inactivation is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Frantz T. A., Peterson D. M., Durbin R. D. Sources of ammonium in oat leaves treated with tabtoxin or methionine sulfoximine. Plant Physiol. 1982 Feb;69(2):345–348. doi: 10.1104/pp.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Determination of submicro quantities of ammonia. Anal Biochem. 1970 Feb;33(2):335–340. doi: 10.1016/0003-2697(70)90304-0. [DOI] [PubMed] [Google Scholar]
- Meek T. D., Villafranca J. J. Kinetic mechanism of Escherichia coli glutamine synthetase. Biochemistry. 1980 Nov 25;19(24):5513–5519. doi: 10.1021/bi00565a008. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. D., Langston-Unkefer P. J., Uchytil T. F., Durbin R. D. Inhibition of Glutamine Synthetase from Pea by Tabtoxinine-beta-lactam. Plant Physiol. 1983 Apr;71(4):912–915. doi: 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


