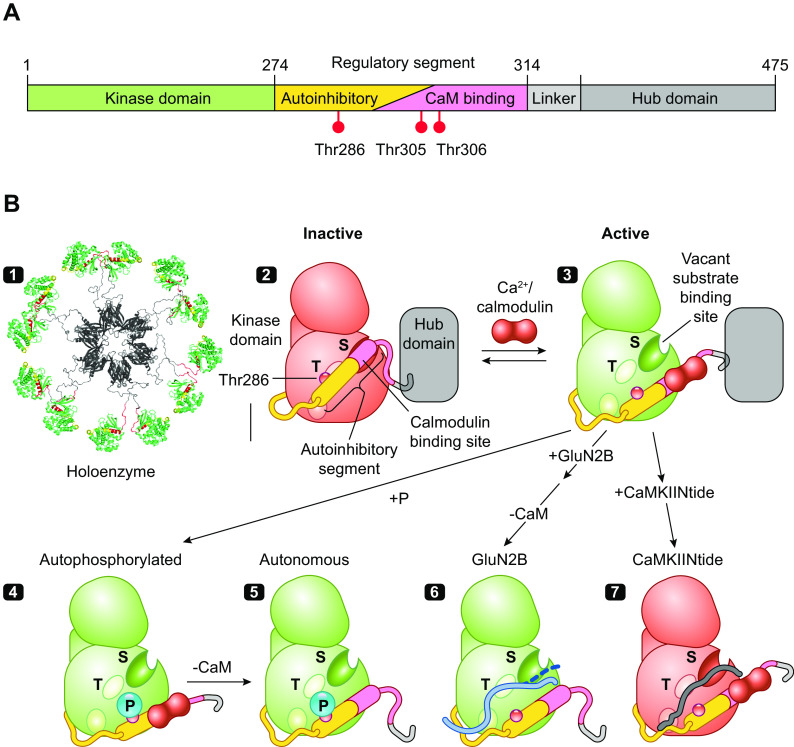
Figure 2.
The structure and regulation of Ca2+/calmodulin (CaM)-dependent protein kinase IIα (CaMKIIα). A: the domain structure of CaMKII with kinase domain followed by regulatory segment, linker, and hub domain. B: schematic diagram showing the structural states of one CaMKII subunit (modified from Ref. 28, per terms of Cold Spring Harbor Perspectives in Biology) 1: The dodecameric structure is formed by hub domains associated as 2 stacked hexameric rings (viewed from above the rings) with pairs of kinase domains from upper and lower ring radiating out via flexible linkers of variable size (PDB: 5U6Y). 2: CaMKII is inactive in the basal state because of conformational and steric effects of its autoinhibitory segment. 3: Ca2+/CaM binding activates CaMKII by competing with kinase domain for binding of the inhibitory regulatory segment, thereby activating the kinase and exposing a surface grove with 3 docking sites (shown as oval surface indentations), including the substrate binding pocket (S) and the general area where T286 was buried (T) that with the third site enables binding of GluN2B and some substrates and inhibitors. 4 and 5: Active CaMKII can then be subject to several modifications or interactions, including autophosphorylation at T286 (4) and dissociation of Ca2+/CaM, leaving a T286P kinase that remains active (autonomous activity) (5). 6: The activated state (autophosphorylated or not) can bind the COOH-terminal tail of GluN2B on a surface groove and T site previously occupied by the regulatory segment (61). GluN2B-bound kinase, measured after removal of Ca2+/CaM, remains active, suggesting only weak binding of COOH-terminal tail at the S site (dashed line). 7: By contrast, CaMKIINtides bind across the entire groove, including the S site (62) to inhibit the kinase.