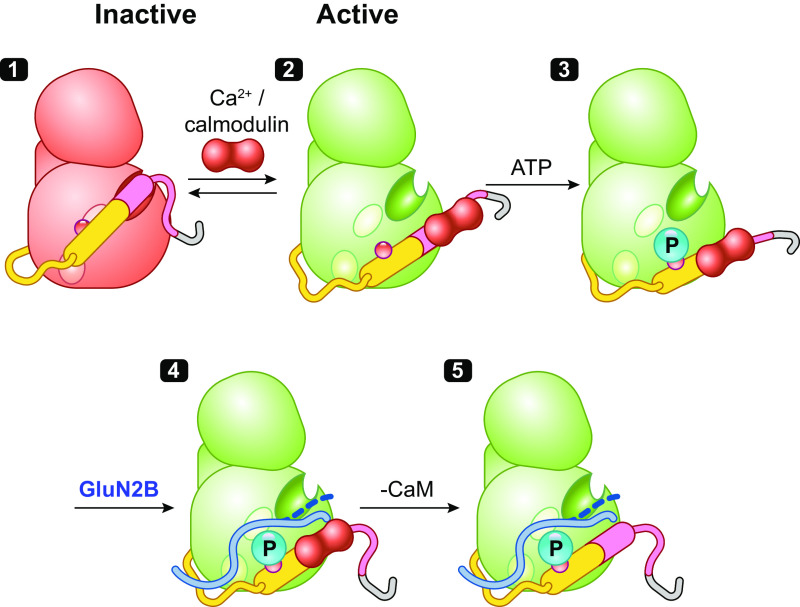
Figure 13.
Proposed sequence of events underlying activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). We next turn to how the CaMKII-GluN2B complex enhances synaptic transmission. Three models are discussed in this review (see FIGURE 7). The first model (receptor-centric model) posits that CaMKII phosphorylates the COOH-terminal domain (CTD) of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and/or transmembrane AMPAR-regulatory protein (TARPs), which are then captured in the postsynaptic density (PSD). The second model (PSD-centric model) proposes that CaMKII modifies component(s) in the PSD creating slots, perhaps unmasking PSD-95 and then allowing the capture of passively diffusing unaltered AMPAR/TARPs. The third model (vesicle-centric model) proposes that CaMKII initiates, by mechanisms that have yet to be fully elucidated, exocytosis of AMPAR-containing vesicles. Although there is an undeniable bias in this review for the PSD-centric model, these models are certainly not mutually exclusive. It should be pointed out that, although it is well established that CaMKII phosphorylates numerous synaptic proteins (66, 434–437), as reviewed here, despite intensive research an essential role for CaMKII phosphorylation of many of these targets largely remains elusive. Perhaps these negative results are telling us something.