Figure 14.
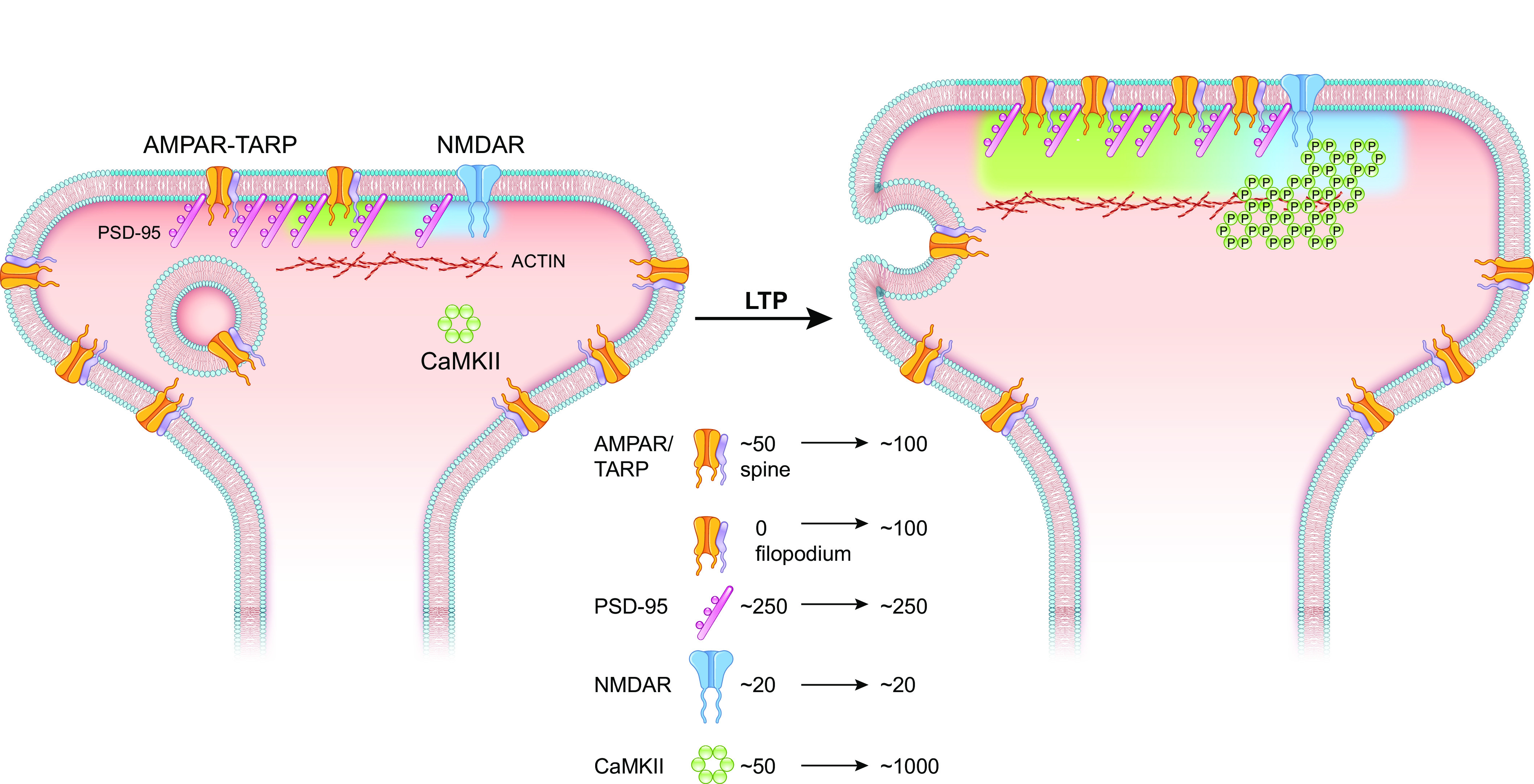
The proposed sequence of events underlying long-term potentiation (LTP). This figure focuses on spine synapses, but a substantial number of synapses are formed on filopodia and assumed to be like spine synapses but are lacking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)/transmembrane AMPAR-regulatory proteins (TARPs). The diagram does not accurately portray the stoichiometry of the various proteins, and actin is distributed throughout the spine. In the resting state the number of PSD-95 molecules outnumbers the AMPAR/TARPs. An LTP-inducing stimulus activates N-methyl-d-aspartate receptors (NMDARs), causing a rise in spine Ca2+. Ca2+ via binding to calmodulin (CaM) activates Ca2+/CaM-dependent protein kinase II (CaMKII). This results in self-association and the binding to the COOH-terminal domain (CTD) of GluN2B. The CaMKII-GluN2B complex is proposed to undergo phase separation (light blue shaded area), which results in a distinct phase separation of AMPAR/TARPs and PSD-95 (light green shaded area). This may be the mechanism enabling PSD-95 to capture AMPARs that normally diffuse into and out of the synapse. This process may be augmented by the exocytosis of AMPAR-containing vesicles. Accompanying these changes is an enlargement of the spine. Most important is a widening of the spine neck, which will have consequences for both chemical and electrical compartmentalization.
