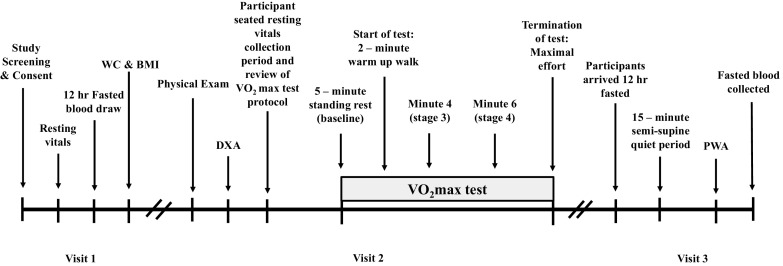
Figure 1.
Study timeline. Participants arrived at the Clinical Research Center (CRC) between 6:00 am and 12:00 pm to complete study visits. Visit 1 included review of the written and verbal informed consent. Upon participant agreement to enroll in the study, resting vitals (i.e., heart rate, blood pressure, and resting electrocardiogram), fasting biochemistries, waist circumference (WC), and body mass index (BMI), were obtained to confirm eligibility. Visit 2 included physical examination completed by the study physician that was then followed by completion of dual X-ray (DXA) assessment and maximal aerobic fitness assessment (V̇o2max). Visit 3 included a resting pulse waveform analysis (PWA) measurement in the semi-supine position and additional blood work.