Abstract
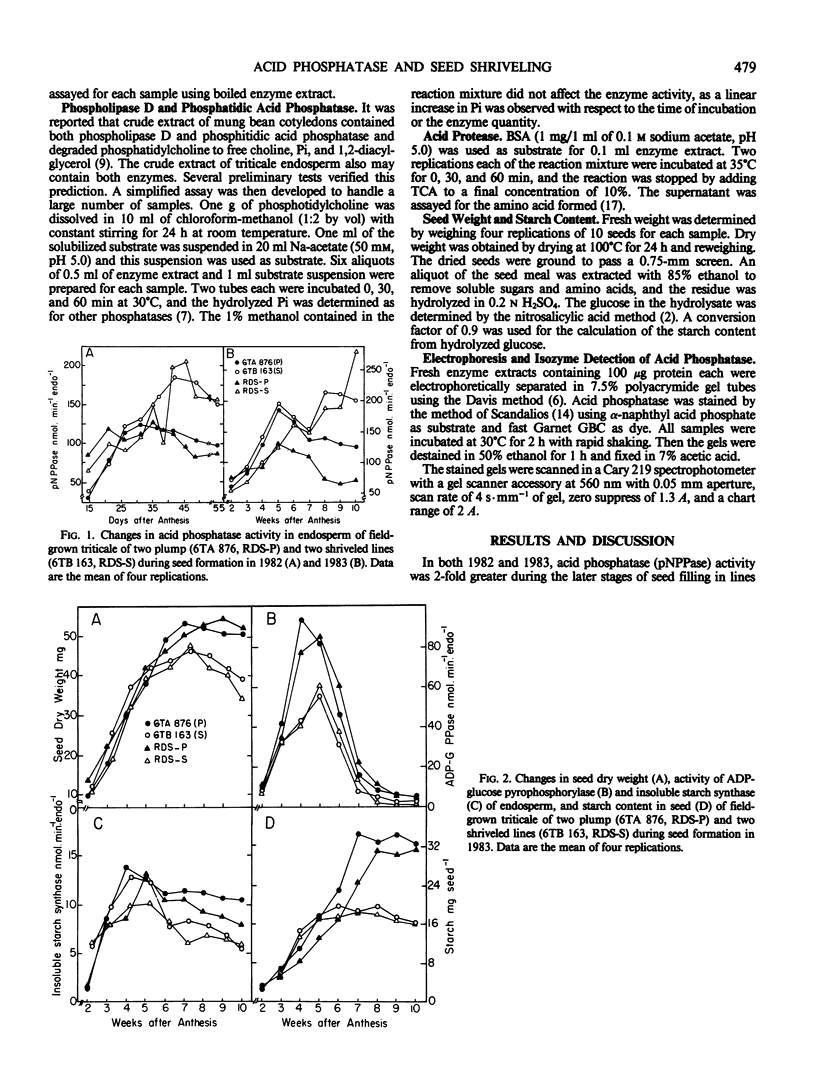
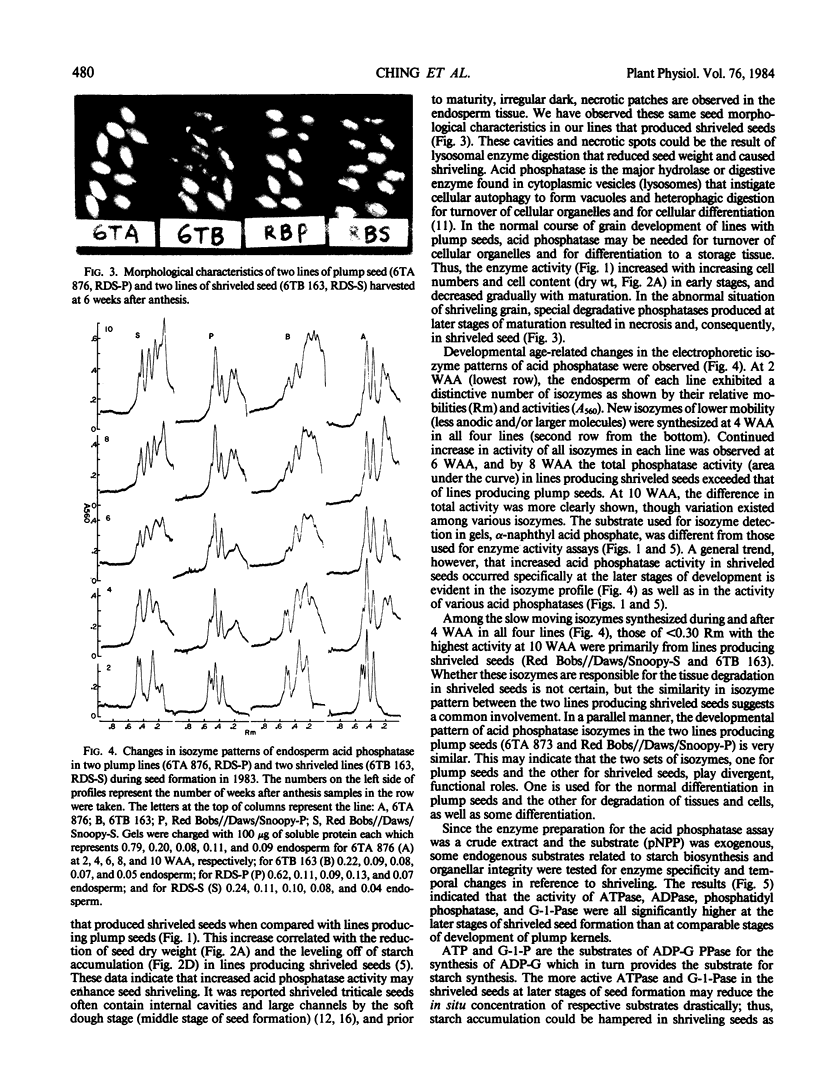
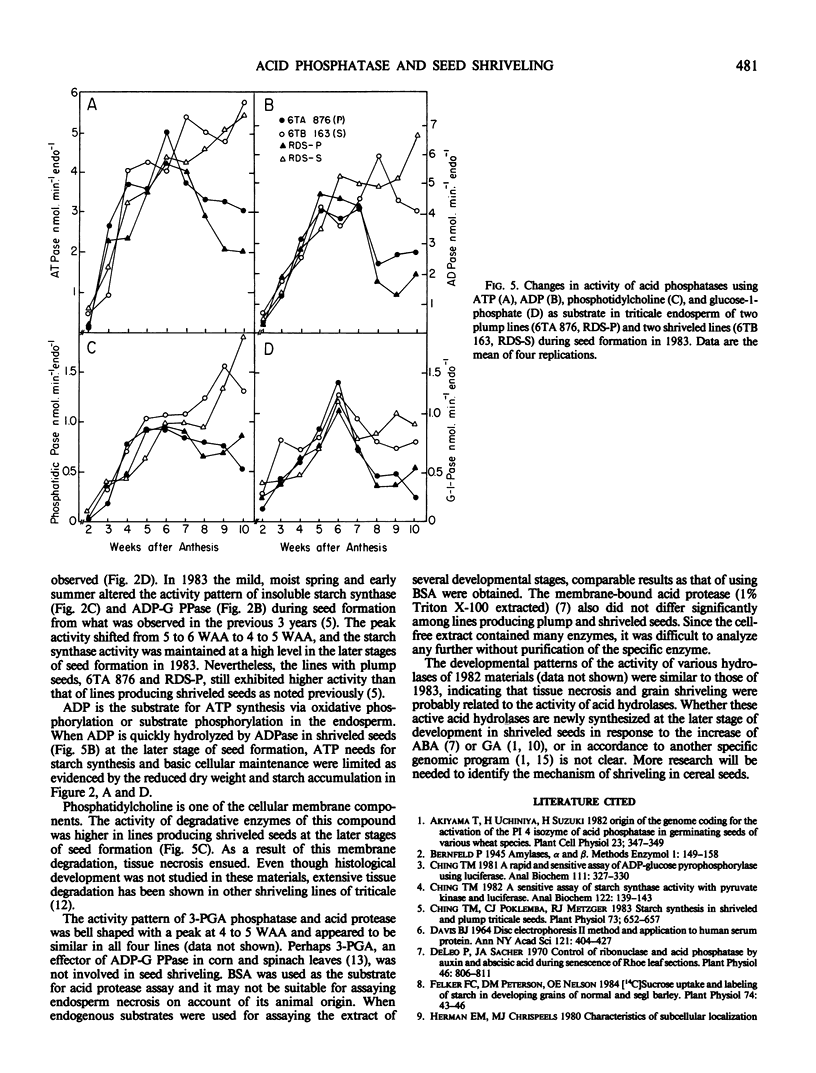
Seed shriveling in the man-made intergeneric hybrid, triticale (x Triticosecale Wittmack) appears to be related to increased activity of endosperm acid phosphatases including para-nitrophenyl phosphatase, ATPase, ADPase, phosphatidic phosphatase, and glucose-1-phosphatase that occur specifically at later stages of seed development. These hydrolases may reduce endogenous substrates for starch synthesis, deplete energy supply for maintenance and biosynthesis of tissue growth, and deassemble membrane structures resulting in a partially filled endosperm and localized necrosis. Electrophoretic isozyme patterns of endosperm acid phosphatase exhibited distinctive differences between lines producing plump and shriveled seeds indicating a divergent role of the isozymes in these two different seed conformations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ching T. M. A sensitive and simple assay of starch synthase activity with pyruvate kinase and luciferase. Anal Biochem. 1982 May 1;122(1):139–143. doi: 10.1016/0003-2697(82)90262-7. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Poklemba C. J., Metzger R. J. Starch synthesis in shriveled and plump triticale seeds. Plant Physiol. 1983 Nov;73(3):652–657. doi: 10.1104/pp.73.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo P., Sacher J. A. Control of ribonuclease and acid phosphatase by auxin and abscisic acid during senescence of Rhoeo leaf sections. Plant Physiol. 1970 Dec;46(6):806–811. doi: 10.1104/pp.46.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker F. C., Peterson D. M., Nelson O. E. [C]Sucrose Uptake and Labeling of Starch in Developing Grains of Normal and segl Barley. Plant Physiol. 1984 Jan;74(1):43–46. doi: 10.1104/pp.74.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. M., Chrispeels M. J. Characteristics and subcellular localization of phospholipase d and phosphatidic Acid phosphatase in mung bean cotyledons. Plant Physiol. 1980 Nov;66(5):1001–1007. doi: 10.1104/pp.66.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. C. Similarities between gibberellins and related compounds in inducing Acid phosphatase and reducing sugar release from barley endosperm. Plant Physiol. 1969 Dec;44(12):1695–1700. doi: 10.1104/pp.44.12.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]



