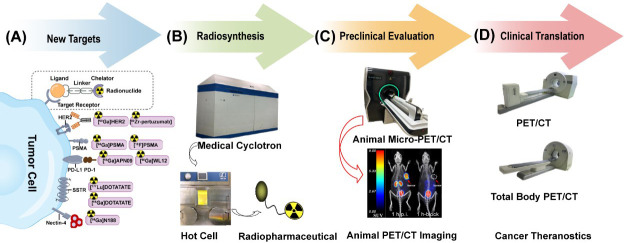
Figure 1.
Radiopharmaceuticals: process from bench to bedside. (A) Radiopharmaceuticals are consisted of four parts including radionuclide, chelator, linker and ligand which could specially bind to cell surface receptors or targets such as PSMA, HER2, PD-1/PD-L1, SSTR and Nectin-4; (B) Preparation of radiopharmaceuticals includes production of nuclides, radiolabeling, and quality control. Animal PET/CT imaging demonstrated high accumulation in tumor region (1); (C) A series of preclinical evaluations are performed to screen out the optimal probes; (D) Optimal probe for clinical translation. Nuclear medicine equipment such as total-body PET/CT is applied to perform whole-body dynamic imaging with quite low doses to achieve visual diagnosis and treatment. PSMA, prostate-specific membrane antigen; HER2, human epidermal growth factor receptor 2; PD-1, programmed death 1; PD-L1, programmed death ligand 1; SSTR, somatostatin receptor; Nectin 4, nectin cell adhesion molecule 4; PET/CT, positron emission tomography/computed tomography.