Abstract
Background
Androgen deprivation therapy (ADT), commonly delivered via a luteinizing hormone-releasing hormone (LHRH) agonist, is the standard treatment for advanced prostate cancer (PC). While quite effective, it has been associated with an increased risk of major adverse cardiovascular events (MACE). The exact mechanisms are not clear. However, it has been theorized that follicle-stimulating hormone (FSH), a pituitary hormone that is involved in controlling normal testosterone levels, which is decreased with LHRH-agonist therapy, may be the culprit. We performed a retrospective population-level study to test the link of FSH levels on the development of MACE, castrate-resistant PC (CRPC), and death among men starting ADT.
Methods
All men (n=1,539) who had an FSH level between 1999 and 2018 within 2 years prior to starting ADT and complete data were identified within the Veterans Affairs (VA) Health System. FSH was dichotomized as low/normal (≤8 IU/mL) and high (>8 IU/mL), using established cut-points. The associations between FSH and time to MACE, death, and CRPC were tested using log-rank tests and multivariable Cox proportional hazards models.
Results
Patients with high FSH were older (median 76 vs. 73 years, P<0.001), started ADT earlier (median 2007 vs. 2009, P=0.027), and had lower body mass index (BMI) (median 29.1 vs. 30.1 kg/m2, P=0.004) compared to those with low/normal FSH. On multivariable analysis, there was no association between FSH and time from ADT to MACE, CRPC, or death.
Conclusions
In this population-level study of men receiving an FSH test prior to starting ADT, there was no association between FSH levels and time from ADT to MACE, CRPC, or death. Although further studies are needed, these results do not support a link between pre-ADT FSH and long-term oncological or cardiovascular outcomes.
Keywords: Prostate cancer, follicle-stimulating hormone (FSH), overall survival (OS)
Highlight box.
Key findings
• We found no association between pre-androgen deprivation therapy (ADT) follicle-stimulating hormone (FSH) levels with cardiovascular or oncological outcomes.
What is known and what is new?
• Only one prior study analyzed FSH and cardiovascular outcomes in prostate cancer patients wherein they studied. 492 men undergoing radical prostatectomy and found no link between pre-treatment FSH and cardiovascular outcomes.
• Our data do not support the clinical utility of pre-treatment FSH levels for assessing risk of cardiovascular or oncological outcomes in men about to start ADT.
What is the implication, and what should change now?
• We found that pre-treatment FSH levels are unlikely to be linked with adverse cardiovascular or oncological outcomes. We encourage studies assessing post-treatment FSH levels.
Introduction
Prostate cancer (PC) is strongly androgen-dependent, but other hormones may also play a role in driving PC growth (1). Specifically, the link between PC pathophysiology and multiple endogenous hormones and proteins has been studied, total testosterone, free testosterone, luteinizing hormone (LH), including prostate-specific antigen (PSA), with only a few studies assessing follicle-stimulating hormone (FSH) (2,3). FSH is a pituitary glycoprotein hormone that is crucial for reproduction and sexual development. When gonadotropin-releasing hormone or LH-releasing hormone (GnRH/LHRH) binds to its receptor, it promotes the hypothalamic-pituitary-gonadal axis (HPGA) to control testosterone production through LH and FSH secretion. FSH is also involved in a negative feedback loop where elevated testosterone levels directly negatively feedback on the pituitary, resulting in suppressed FSH levels (4). FSH then stimulates Sertoli cells to make androgen-binding protein as well as maintain normal sperm production (5,6). Nevertheless, apart from the typical release of FSH by the anterior pituitary gland, the prostate gland has been observed to produce FSH and exhibit FSH receptors (FSHR) in pathological conditions such as benign prostatic hyperplasia (BPH) and advanced or metastatic PC (7-9). As a result, there has been an increased interest in examining the involvement of FSH in the progression and onset of PC.
A key treatment for advanced PC involves lowering androgen levels using androgen deprivation therapy (ADT) (10). Given ADT is typically performed by a LHRH agonist, FSH levels will decrease. The consequences of the FSH fall with ADT on oncological outcomes are unclear. As noted, given that PC cells both produce FSH and express FSHR, there is concern that FSH may be acting as an autocrine growth factor for PC. Indeed, direct targeting of FSHR is now being tested as a novel treatment for PC, in addition to potential combination therapies with ADT (11). Moreover, some studies suggest FSH may play a role in the development of atherosclerosis during ADT, a well-known risk of ADT (12-15). There has even been some literature suggesting that regularly monitoring FSH during ADT may have benefits as most PC patients are older men at elevated risk of cardiovascular disease (11). In addition to treatment monitoring, research in the past decade has revealed a correlation between elevated FSH levels and reduced total testosterone levels. Moreover, these increased FSH levels have been linked to more aggressive forms of PC and an increased likelihood of extraprostatic extension (16-18). Intriguingly, a study found that higher FSH after the start of ADT was associated with shorter time to development of castrate-resistant PC (CRPC). However, whether FSH levels prior to ADT hold similar prognostic importance is unknown.
We sought to evaluate FSH levels in PC patients before ADT and assess if there are associations between FSH levels and the development of major adverse cardiovascular events (MACE), CRPC, death. If positive, this would suggest FSH may be a novel biomarker of cardiovascular and oncological outcomes after ADT. Based upon the potential role of FSH in atherosclerosis development, we hypothesized that higher FSH levels prior to ADT are associated with long-term risk of MACE. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-114/rc).
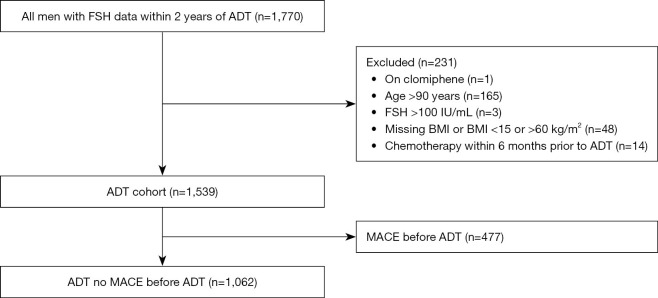
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Durham Veterans Affairs (VA) Central Institutional Review Board (No. 1827) and individual consent for this retrospective analysis was waived. Additionally, the study ensures that all data collected will be maintained with strict confidentiality. The data were retrieved from the corporate data warehouse. Using claims for PC and crossing this with pharmacy records for ADT and those who had a FSH test, we identified 1,770 PC patients who had a pretreatment FSH test between 1999 and 2018 within 2 years prior to starting ADT in the VA Health System (Figure 1). Among these, we excluded men who received clomiphene within a year prior to the FSH test (n=1), >90 years of age (n=165), had an FSH level >100 IU/mL (n=3), had a body mass index (BMI) <15 or >60 kg/m2 or missing (n=48), or had chemotherapy within 6 months of ADT start (n=14), leading to our analysis cohort of 1,539 men to assess the association between serum FSH and overall survival (OS) and CRPC. In analyses to assess the association between serum FSH and MACE development, we further excluded patients who had MACE before ADT (n=477) leading to a cohort of 1,062 patients. FSH was dichotomized a priori as low/normal (≤8 IU/mL) and high (>8 IU/mL) as we previously described (19). Baseline and clinical characteristics were compared between the FSH groups (low/normal vs. high). Continuous variables were compared using Wilcoxon rank sum test and categorical variables were compared using Chi-Square test.
Figure 1.
Consort diagram for patient selection (ADT cohort). FSH, follicle-stimulating hormone; ADT, androgen deprivation therapy; BMI, body mass index; MACE, major adverse cardiovascular events.
MACE was defined as myocardial infarction, coronary artery disease, cardiovascular disease, congestive heart disease or stroke based upon the International Classification of Diseases (ICD)-9 and ICD-10 claims. CRPC was determined by querying lab and prescription data to identify PSA rise while on continuous ADT.
The association between serum FSH and MACE development was the primary outcome. Secondary outcomes were the associations between serum FSH and CRPC and OS. Time from ADT to events of interest (MACE, death, or CRPC) was estimated by Kaplan-Meier method and differences between FSH groups were tested using the log-rank test. Univariable and multivariable Cox proportional hazards (CPH) models were used to assess the association between serum FSH and all outcomes. Multivariable models were adjusted for age, year, race, BMI, Charlson Comorbidity Index (CCI), primary treatment, biopsy grade group, PSA, time from FSH to ADT, and chemotherapy (time-dependent). The model assessing MACE as an outcome was, in addition, adjusted for testosterone level (lowest, middle, and highest tertile vs. missing). CPH assumption was assessed using Schoenfeld residuals and for all outcomes CPH assumptions were met. For analyses, we defined the start of follow-up as the time of ADT initiation. In analysis, FSH was modeled as continuous, log continuous, and categorical variables, though the dichotomous analyses being considered primary.
Statistical analyses
Statistical analyses were conducted using SAS Version 9.4 (SAS institute, Cary, NC, USA) and Stata 14.2 (Stata Corp., College Station, Texas, USA). All significance tests were two-sided with P<0.05.
Results
Of the 1,539 patients, 878 (57%) had a low/normal FSH level and 661 (43%) had a high level. Patients with high FSH were older (median 76 vs. 73 years, P<0.001), started ADT earlier (median 2007 vs. 2009, P=0.027), and had a lower BMI (median 29.1 vs. 30.1 kg/m2, P=0.004) compared to those with low/normal FSH. Patients with low/normal vs. high FSH were comparable by race, primary treatment type [radiation therapy (XRT) or radical prostatectomy (RP)], grade group on diagnostic biopsy, PSA and Charlson Comorbidity Index (CCI) at ADT, as well as testosterone and chemotherapy use (all P≥0.053) (Table 1). After excluding men who had MACE prior to ADT, similar results were seen in that patients with high FSH were older (median 76 vs. 72 years, P<0.001), had lower BMI (median 28.7 vs. 29.5 kg/m2, P=0.010) and were likewise comparable on race, treatment type, grade group, PSA and CCI at ADT, as well as testosterone and chemotherapy use (P≥0.095) (Table 2).
Table 1. Characteristics of patients who received ADT, stratified by FSH.
| Demographic variables | FSH | P value | |
|---|---|---|---|
| Low/normal (≤8 UI/mL) (N=878) | High (>8 UI/mL) (N=661) | ||
| Age at ADT (years) | 73 [68, 79] | 76 [70, 83] | <0.0011 |
| Year of ADT start | 2009 [2004, 2013] | 2007 [2004, 2012] | 0.0271 |
| Race, n [%] | 0.1162 | ||
| Black | 307 [35] | 195 [30] | |
| White | 473 [54] | 377 [57] | |
| Other | 40 [5] | 34 [5] | |
| Unknown | 58 [7] | 55 [8] | |
| BMI at ADT (kg/m2) | 30.1 (26.3, 34.2) | 29.1 (25.8, 33.2) | 0.0041 |
| CCI at ADT | 0.1022 | ||
| 0–2 | 252 [29] | 165 [25] | |
| 3+ | 626 [71] | 496 [75] | |
| Primary XRT or RP | 111 [13] | 98 [15] | 0.2162 |
| Grade group | 0.3472 | ||
| 1 | 201 [23] | 144 [22] | |
| 2–3 | 286 [33] | 201 [30] | |
| 4–5 | 275 [31] | 208 [31] | |
| Unknown | 116 [13] | 108 [16] | |
| PSA at ADT (ng/mL) | 6.6 (2.5, 13.9) | 6.5 (2.4, 13.8) | 0.8071 |
| Testosterone pre-ADT | 0.6222 | ||
| <30 ng/mL | 183 [21] | 136 [21] | |
| 30–299 ng/mL | 203 [23] | 138 [21] | |
| ≥300 ng/mL | 182 [21] | 152 [23] | |
| Missing | 310 [35] | 235 [36] | |
| Chemo before ADT | 0.0532 | ||
| Never | 753 [86] | 579 [88] | |
| Before ADT | 18 [2] | 22 [3] | |
| After ADT | 107 [12] | 60 [9] | |
| Months from FSH to ADT | 8.4 (3.0, 15.9) | 7.9 (3.2, 14.6) | 0.1991 |
| CRPC | 231 [26] | 177 [27] | 0.8372 |
| Dead | 381 [43] | 337 [51] | 0.0032 |
Data are shown as median (Q1, Q3) or n [%]. 1, Wilcoxon; 2, Chi-Square. ADT, androgen deprivation therapy; FSH, follicle-stimulating hormone; BMI, body mass index; CCI, Charlson Comorbidity Index; XRT, radiation therapy; RP, radical prostatectomy; PSA, prostatic-specific antigen; CRPC, castration-resistant prostate cancer.
Table 2. Characteristics of patients who received ADT and no MACE before ADT, stratified by FSH.
| Demographic variables | FSH | P value | |
|---|---|---|---|
| Low/normal (≤8 UI/mL) (N=620) | High (>8 UI/mL) (N=442) | ||
| Age at ADT (years) | 72 [67, 78] | 76 [70, 82] | <0.0011 |
| Year of ADT start | 2008 [2004, 2013] | 2007 [2004, 2012] | 0.1201 |
| Race | 0.4842 | ||
| Black | 221 [36] | 141 [32] | |
| White | 327 [53] | 243 [55] | |
| Other | 26 [4] | 25 [6] | |
| Unknown | 46 [7] | 33 [7] | |
| BMI at ADT (kg/m2) | 29.5 (26.0, 33.6) | 28.7 (25.3, 32.4) | 0.0101 |
| CCI at ADT | 0.0952 | ||
| 0–2 | 227 [37] | 140 [32] | |
| 3+ | 393 [63] | 302 [68] | |
| Primary XRT or RP | 81 [13] | 67 [15] | 0.3312 |
| Grade group | 0.8892 | ||
| 1 | 135 [22] | 104 [24] | |
| 2–3 | 204 [33] | 138 [31] | |
| 4–5 | 191 [31] | 134 [30] | |
| Unknown | 90 [15] | 66 [15] | |
| PSA at ADT (ng/mL) | 6.9 (2.6, 15.5) | 7.0 (2.8, 14.2) | 0.9291 |
| Testosterone pre-ADT | 0.1552 | ||
| <30 ng/mL | 131 [21] | 92 [21] | |
| 30–299 ng/mL | 143 [23] | 80 [18] | |
| ≥300 ng/mL | 134 [22] | 115 [26] | |
| Missing | 212 [34] | 155 [35] | |
| Chemo before ADT | 0.2041 | ||
| Never | 526 [85] | 386 [87] | |
| Before ADT | 12 [2] | 15 [3] | |
| After ADT | 82 [13] | 41 [9] | |
| Months from FSH to ADT | 8.1 (2.7, 15.8) | 7.8 (2.8, 14.1) | 0.2111 |
| MACE | 162 [26] | 120 [27] | 0.7112 |
Data are shown as median (Q1, Q3) or n [%]. 1, Wilcoxon; 2, Chi-Square. ADT, androgen deprivation therapy; MACE, major adverse cardiovascular events; FSH, follicle-stimulating hormone; BMI, body mass index; CCI, Charlson Comorbidity Index; XRT, radiation therapy; RP, radical prostatectomy; PSA, prostatic-specific antigen.
Time to MACE from ADT was no different between the FSH groups (log-rank P=0.43) (Figure 2). Regardless of FSH modeling as continuous, log continuous, or categorical variables, both univariable and multivariable Cox regression analyses showed no association between FSH levels and the development of MACE among men undergoing ADT (HR 1.00–1.10, P≥0.43) (Table 3).
Figure 2.
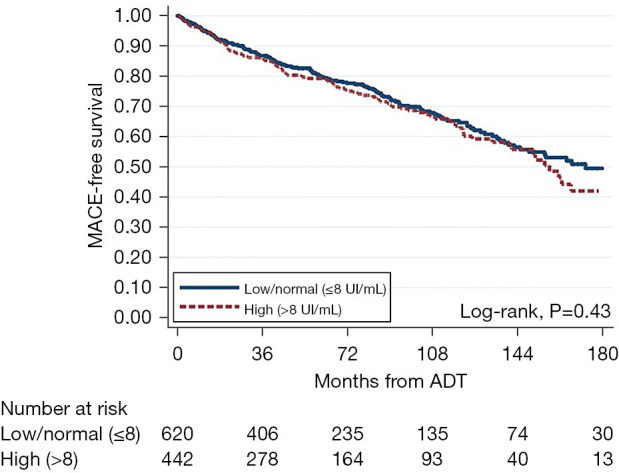
Kaplan-Meier curve for time from ADT to MACE, stratified by FSH level. MACE, major adverse cardiovascular events; ADT, androgen deprivation therapy; FSH, follicle-stimulating hormone.
Table 3. Association between first FSH test and time from ADT to MACE.
| FSH measures | Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| FSH—continuous | 1.00 | 0.99–1.01 | 0.65 | 1.00 | 0.98–1.01 | 0.62 | |
| FSH—log continuous | 1.05 | 0.89–1.24 | 0.60 | 1.00 | 0.83–1.21 | 0.98 | |
| FSH—categorical | |||||||
| Low/normal (≤8 UI/mL) | Ref. | – | – | Ref. | – | – | |
| High (>8 UI/mL) | 1.10 | 0.87–1.39 | 0.43 | 1.04 | 0.81–1.34 | 0.74 | |
*, adjusted for age, year, race, BMI, Charlson Comorbidity Index, primary treatment, biopsy grade group, PSA, time from FSH to ADT, testosterone and chemotherapy (time-dependent). FSH, follicle-stimulating hormone; ADT, androgen deprivation therapy; MACE, major adverse cardiovascular events; HR, hazard ratio; CI, confidence interval; Ref., reference; BMI, body mass index; PSA, prostatic-specific antigen.
Time from ADT to CRPC was not significantly different between the two FSH groups (log-rank P=0.636) (Figure 3). Regardless of FSH modeling as continuous, log continuous, or categorical variables, both univariable and multivariable Cox regression analyses showed no association between FSH levels and CRPC (Table 4).
Figure 3.
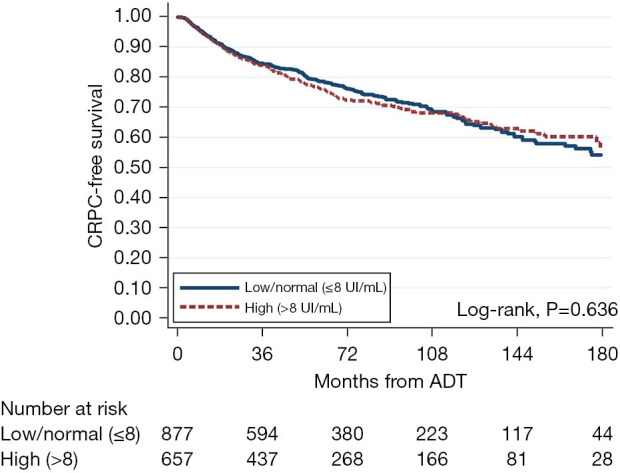
Kaplan-Meier curve for time from ADT to CRPC, stratified by FSH level. CRPC, castration-resistant prostate cancer; ADT, androgen deprivation therapy; FSH, follicle-stimulating hormone.
Table 4. Association between first FSH test and time from ADT to CRPC.
| FSH measures | Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| FSH—continuous | 1.01 | 0.99–1.02 | 0.09 | 1.00 | 0.99–1.01 | 0.77 | |
| FSH—log continuous | 1.09 | 0.95–1.25 | 0.22 | 0.98 | 0.85–1.13 | 0.80 | |
| FSH—categorical | |||||||
| Low/normal (≤8 UI/mL) | Ref. | – | – | Ref. | – | – | |
| High (>8 UI/mL) | 1.05 | 0.86–1.43 | 0.64 | 0.94 | 0.77–1.16 | 0.57 | |
*, adjusted for age, year, race, BMI, Charlson Comorbidity Index, primary treatment, biopsy grade group, PSA, time from FSH to ADT, and chemotherapy (time-dependent). FSH, follicle-stimulating hormone; ADT, androgen deprivation therapy; CRPC, castration-resistant prostate cancer; HR, hazard ratio; CI, confidence interval; Ref., reference; BMI, body mass index; PSA, prostate-specific antigen.
In univariable analysis, higher FSH was associated with worse OS when assessed as a continuous and log-transformed variable (HR 1.01, 95% CI: 1.01–1.02; HR 1.23, 95% CI: 1.11–1.37, respectively) (Figure 4, Table 5). Similarly, when modeled as a categorical variable, patients with high (>8 UI/mL) FSH values were at increased risk of death compared to those in the low/normal category (HR 1.22, 95% CI: 1.05–1.41). After multivariable adjustment, however, no association was found with OS, regardless of whether FSH was assessed as a continuous, log continuous, or categorical variable.
Figure 4.
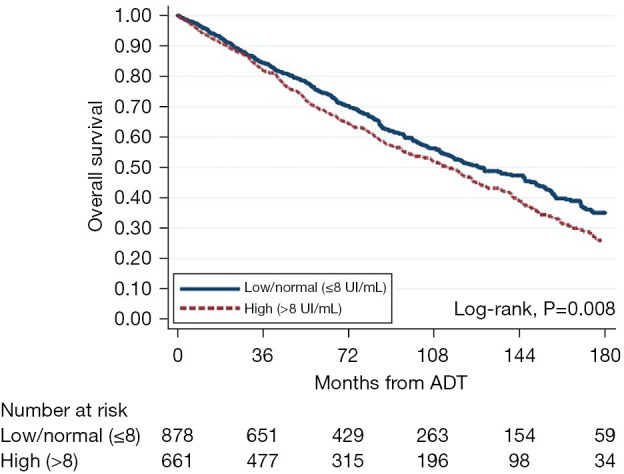
Kaplan-Meier curve for time from ADT to mortality, stratified by FSH level. ADT, androgen deprivation therapy; FSH, follicle-stimulating hormone.
Table 5. Association between first FSH test and time from ADT to all-cause mortality.
| FSH measures | Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| FSH—continuous | 1.01 | 1.01–1.02 | <0.001 | 1.01 | 0.99–1.01 | 0.10 | |
| FSH—log continuous | 1.23 | 1.11–1.37 | <0.001 | 1.04 | 0.93–1.16 | 0.47 | |
| FSH—categorical | |||||||
| Low/normal (≤8 UI/mL) | Ref. | – | – | Ref. | – | – | |
| High (>8 UI/mL) | 1.22 | 1.05–1.41 | 0.008 | 1.03 | 0.88–1.20 | 0.70 | |
*, adjusted for age, year, race, BMI, Charlson Comorbidity Index, primary treatment, biopsy grade group, PSA, time from FSH to ADT, and chemotherapy (time-dependent). FSH, follicle-stimulating hormone; ADT, androgen deprivation therapy; HR, hazard ratio; CI, confidence interval; Ref., reference; BMI, body mass index; PSA, prostate-specific antigen.
Discussion
Androgen-deprivation therapy is the mainstay treatment for metastatic and CRPC (20). However, while life-prolonging in the right situation, ADT poses several side effects and risks including changes in body composition, increased fracture risk, development of insulin resistance, unfavorable lipid profile, and increased cardiovascular risk (21). The exact mechanisms to explain the increased cardiovascular risk are not clear, though some studies suggest elevated FSH levels may mediate some of this risk (12). Based upon the potential role of FSH in atherosclerosis development, we hypothesized that higher FSH levels prior to ADT are associated with long-term risk of MACE. However, to date, no previous studies assessed the relationship between FSH levels prior to ADT to MACE outcomes. To address this, we investigated the association of FSH levels prior to ADT with the development of MACE, CRPC, or death within the VA medical records. Using data from over 1,500 men, we found no association between FSH levels prior to ADT and long-term risk of MACE, CRPC, or death. These data suggest that pre-treatment FSH levels are unlikely to be linked with adverse cardiovascular or oncological outcomes.
To our knowledge, the current study is the first study analyzing a cohort of men all undergoing ADT assessing pre-ADT FSH levels as risk factors for cardiovascular outcomes. To date, only one prior study analyzed FSH and cardiovascular outcomes in PC patients. In that study, among 492 men undergoing RP, there was no link between pre-treatment FSH and cardiovascular outcomes (22). Combined with the current study, where we also found no association between FSH and MACE, we conclude that the current literature does not support pre-treatment FSH levels predicting cardiovascular outcomes in men with PC. Whether FSH levels after treatment with ADT would show similar null associations requires further study.
In contrast to cardiovascular outcomes, which has been examined in only one study to date, the association between FSH and oncological outcomes has been studied in several papers. A retrospective analysis of 96 patients undergoing RP found significant correlation between preoperative FSH levels and extraprostatic extension (17). In contrast, a study of 126 patients undergoing RP found no association between preoperative FSH levels and tumor volume (18). Consistent with this latter study, we found null associations between pre-treatment FSH groups and oncological outcomes, albeit among men undergoing ADT. Intriguingly, one study examined FSH levels after starting ADT among 103 men and found that higher FSH levels were correlated with shorter time to CRPC (23). Collectively, these data suggest that pre-treatment FSH levels may have limited prognostic value, whereas as post-treatment values after ADT may have value, though this requires further study.
In men with PC, the key causes of death are cardiovascular and PC (24). Given null associations between FSH and these outcomes, it is not surprising we found no association between pre-treatment FSH and OS. These data are similar to results from others that also found no association between FSH and OS (25). As noted above, whether post-ADT FSH levels would give similar results requires further study.
Our study is not devoid of limitations. First, similar to any observational study, these findings do not indicate the lack of cause-and-effect connection between FSH and the development of MACE, CRPC, or death; rather FSH and these outcomes were merely not associated. Additionally, while our cutoff for low/normal and high FSH values was as we did in the past and this was selected a priori, the exact cut-offs for cardiovascular and oncological outcomes may differ. However, even when analyzed as continuous or log-continuous variable, FSH was unrelated to these outcomes. It is possible further analyses may find alternative cut-points that are linked with these outcomes. We did not perform such analyses as there was no strong rationale that alternative cut-offs would be linked with these outcomes and exploring various cut-offs without pre-defining them, would be viewed as multiple testing and hypothesis generating vs. our current study which aimed to test the hypothesis that FSH was linked with these outcomes. Third, a sizable percent of men were missing testosterone levels at the time of FSH testing preventing us from analyzing how these values may have modified the associations between FSH and MACE, CRPC, or OS. Fourth, given the study was population-level with very large numbers, we could not abstract Gleason score and were thus not able to adjust for grade in our multivariable models. Fifth, we did not report drinking history, as it was not available within our dataset. Consequently, the potential influence of drinking history on the outcome of MACE in our study remains unknown, thereby warranting further investigation. Sixth, it is likely that many factors influence FSH levels that we could not capture or control for, and it is possible that the links we found may be driven by these factors rather than FSH itself. Indeed, given that FSH is not a commonly ordered test, even the exact reason FSH was ordered was unknown. Finally, our analyses only included FSH levels prior to ADT. Given these limitations, future studies including a prospective evaluation with serial FSH levels, including post-treatment FSH levels may provide further granularity regarding associations between FSH levels and the development of MACE, CRPC, or death.
Conclusions
To our knowledge, the current study is the first study analyzing a cohort of men all undergoing ADT assessing pre-treatment FSH levels as risk factors for cardiovascular outcomes. We found no association between pre-ADT FSH levels with cardiovascular or oncological outcomes. As such, these data do not support the clinical utility of pre-treatment FSH levels in these men and do not support the hypothesis that FSH is linked with cardiovascular outcomes. Further studies assessing post-treatment FSH levels are needed.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This research received funding from Ferring Pharmaceuticals Inc. This work was funded by NIH (No. T32CA240172-04, to Nadine A. Friedrich, MD).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Durham VA Central Institutional Review Board (No. 1827) and individual consent for this retrospective analysis was waived. Additionally, the study ensures that all data collected will be maintained with strict confidentiality.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-114/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-114/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-114/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-114/coif). SJF serves as an unpaid editorial board member of Translational Andrology and Urology from August 2023 to July 2025 and is a consultant for Myovant, Pfizer, Astellas, Bayer, Janssen, Sanori, Merck, and Astra Zeneca. NAF reports the funding from NIH (No. T32CA240172-04). This study was funded by Ferring Pharmaceuticals Inc. The authors have no other conflicts of interest to declare.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9-12. 10.1016/S0022-5347(05)64820-3 [DOI] [PubMed] [Google Scholar]
- 2.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 1987;317:909-16. 10.1056/NEJM198710083171501 [DOI] [PubMed] [Google Scholar]
- 3.Stenman UH, Abrahamsson PA, Aus G, et al. Prognostic value of serum markers for prostate cancer. Scand J Urol Nephrol Suppl 2005;(216):64-81. 10.1080/03008880510030941 [DOI] [PubMed] [Google Scholar]
- 4.Bagatell CJ, Dahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Androl 1994;15:15-21. [PubMed] [Google Scholar]
- 5.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev 2001;22:764-86. 10.1210/edrv.22.6.0446 [DOI] [PubMed] [Google Scholar]
- 6.Porter AT, F A C R O, Ben-Josef E. Humoral mechanisms in prostate cancer: A role for FSH. Urol Oncol 2001;6:131-8. 10.1016/S1078-1439(00)00124-1 [DOI] [PubMed] [Google Scholar]
- 7.Garde SV, Sheth AR, Shah MG, et al. Prostate--an extrapituitary source of follicle-stimulating hormone (FSH): occurrence, localization, and de novo biosynthesis and its hormonal modulation in primates and rodents. Prostate 1991;18:271-87. 10.1002/pros.2990180402 [DOI] [PubMed] [Google Scholar]
- 8.Mariani S, Salvatori L, Basciani S, et al. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J Urol 2006;175:2072-7; discussion 2077. 10.1016/S0022-5347(06)00273-4 [DOI] [PubMed] [Google Scholar]
- 9.Dizeyi N, Trzybulska D, Al-Jebari Y, et al. Cell-based evidence regarding the role of FSH in prostate cancer. Urol Oncol 2019;37:290.e1-8. 10.1016/j.urolonc.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 10.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin 2002;52:154-79. 10.3322/canjclin.52.3.154 [DOI] [PubMed] [Google Scholar]
- 11.Crawford ED, Rove KO, Schally AV, et al. The role of the FSH system in the development and progression of prostate cancer. American Journal of Hematology/Oncology 2014;10:5-13. [Google Scholar]
- 12.Crawford ED, Schally AV, Pinthus JH, et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol 2017;35:183-91. 10.1016/j.urolonc.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 13.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448-56. 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 14.Keating NL, O'Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010;102:39-46. 10.1093/jnci/djp404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis 2019;22:24-38. 10.1038/s41391-018-0079-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoare D, Skinner TA, Black A, et al. Serum follicle-stimulating hormone levels predict time to development of castration-resistant prostate cancer. Can Urol Assoc J 2015;9:122-7. 10.5489/cuaj.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ide H, Terado Y, Sakamaki K, et al. Serum level of follicle-stimulating hormone is associated with extraprostatic extension of prostate cancer. Prostate Int 2013;1:109-12. 10.12954/PI.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porcaro AB, Siracusano S, de Luyk N, et al. Simultaneous Measurements of Follicle Stimulating Hormone and Total Testosterone and Associations in Clinically Localized Prostate Cancer. Curr Urol 2017;10:174-81. 10.1159/000447177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dymanus K, Howard LE, Oyekunle T, et al. Are higher pre-diagnosis follicle stimulating hormone levels associated with long-term prostate cancer risk? Prostate 2022;82:1558-63. 10.1002/pros.24429 [DOI] [PubMed] [Google Scholar]
- 20.Kessel A, Kohli M, Swami U. Current management of metastatic castration-sensitive prostate cancer. Cancer Treat Res Commun 2021;28:100384. 10.1016/j.ctarc.2021.100384 [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Freedland SJ. Androgen deprivation therapy in prostate cancer: anticipated side-effects and their management. Curr Opin Support Palliat Care 2010;4:147-52. 10.1097/SPC.0b013e32833bd913 [DOI] [PubMed] [Google Scholar]
- 22.Kourbanhoussen K, Joncas FH, D, Wallis CJ, et al. Follicle-stimulating hormone (FSH) levels prior to prostatectomy are not related to long-term oncologic or cardiovascular outcomes for men with prostate cancer. Asian J Androl 2022;24:21-5. 10.4103/aja.aja_58_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinthus JH. Follicle-stimulating hormone: A potential surrogate marker for androgen deprivation therapy oncological and systemic effects. Can Urol Assoc J 2015;9:E226-E227. 10.5489/cuaj.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong DP, Fradet V, Shayegan B, et al. Cardiovascular Risk in Men with Prostate Cancer: Insights from the RADICAL PC Study. J Urol 2020;203:1109-16. 10.1097/JU.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 25.Atchia K, Joncas FH, Trasiewicz LS, et al. Follicle Stimulating Hormone Levels During Androgen Deprivation Therapy Are Not Associated With Survival or Development of Castration-Resistant Prostate Cancer. Société Internationale d'Urologie Journal 2022;3:56-61. 10.48083/LWHQ7760 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as