Abstract
Background and objective
Amoebic liver abscess (ALA) and pyogenic liver abscesses (PLA) are the most common causes of liver abscess in developing and developed countries, respectively. Although incidence of liver abscess is low, but mortality is high amongst the patients due to delayed diagnosis. The study was done to find out the prevalence of amoebic and PLA among patients of liver abscess. The clinical, personal, and demographical details were also evaluated to find out the risk factor(s) associated with ALA and PLA, respectively.
Method
A retrospective study was conducted to find the prevalence of amoebic and PLA. Clinical, demographic, personal details were evaluated from hospital records. Laboratory parameters such as total leucocyte count, platelets, bilirubin, ESR (Erythrocyte Sedimentation Rate), hemoglobin, glycosylated hemoglobin (HbA1c), alkaline phosphate (ALP), Aspartate aminotransferase (SGOT/AST), Alanine aminotransferase (SGPT/ALT), serum albumin, bilirubin levels, and procalcitonin were recorded. The Ultrasonography (USG) findings regarding the size, location, volume, and number of abscesses were also analyzed.
Results
Total of 107 patients of liver abscess were evaluated, and 61.6% of patients were of amoebic etiology, and 25.3% were of pyogenic etiology. Males of 20–60 years of age were predominantly affected with right upper quadrant pain and fever as the most common presentations. ALA patients were found to have solitary abscess in the right lobe involving 6th and 7th segments, with decreased hemoglobin, hyperbilirubinemia, elevated ALP and SGOT, with normal SGPT, and addiction to alcohol. PLA patients had increased HbA1c, increased PCT values, low serum albumin levels, and low platelet-to–white blood cell values. The most common bacteria causing PLA was Escherichia coli (n = 8) followed by Enterobacter cloacae (n = 5). Mortality was seen in 6 patients.
Conclusion
Liver abscess is found to have relatively high mortality and morbidity. Therefore, early diagnosis is the only method to prevent mortality and morbidity in these patients. Since the presentation is very nonspecific, evaluation of certain risk factors and laboratory parameters can aid in the diagnosis.
Keywords: amoebic, liver abscess, mortality, pyogenic, right lobe
Highlights
-
•
Of the 107 patients of liver abscess, 61.6% were of amoebic etiology, and 25.3% were of pyogenic etiology.
-
•
Males of 20–60 years of age were predominantly affected.
-
•
ALA patients were found to have solitary abscess in the right lobe involving 6th and 7th segments.
-
•
ALA patients had elevated ALP and SGOT with normal SGPT.
-
•
The most common bacteria causing PLA was Escherichia coli (n = 8) followed by Enterobacter cloacae (n = 5).
A liver abscess is a pus-filled collection in the liver that can have either pyogenic or amoebic etiologies and arises from either liver damage or an intra-abdominal infection that has spread from the portal vein.1 Although liver abscesses are uncommon, they must be promptly identified and treated due to the high risk of death in untreated individuals.2 The annual incidence rate is about 2.3 cases per 100,000 people with males affected more than females.1
The most frequent causes of liver abscess are pyogenic liver abscess (PLA) and amoebic liver abscess (ALA).3 ALA is caused due to consumption of contaminated food and water, where the protozoa penetrate the small intestine to enter the mesenteric vessel and reaches the liver. ALA cases are more common in areas where Entamoeba histolytica is common.4 They account for two-third of the cases of liver abscess in developing countries.3,5,6 The most frequent extra-intestinal symptom of amebiasis is ALA, and liver abscesses develop in 2–5% of cases of intestinal amoebiasis.3 Contrarily, PLA is more prevalent in developed nations, where it accounts for three-fourth of cases of liver abscess.2 They develop secondary to biliary or intestinal tract infections. The current methods for differentiating ALA from PLA include microscopic analysis, serological detection of antiamoebic IgG, and aspirate culture for pyogenic organisms. Even though it confirms the presence of ALA, the finding of trophozoites in the liver aspirate is quite insensitive.7 The serum anti-amoebic IgG antibody is most usually used in the diagnosis of ALA.8 In endemic areas, this assay might not be able to tell the difference between a past and present infection. Also, some healthy individuals may also have anti-amoebic IgG antibodies.9 The mortality rate in ALA patients ranges from 2% to 15%.10,11 Among PLA patients, the mortality ranges from 2% to 8%.12,13 Chemotherapy works effectively to treat ALA, and it is rarely necessary to drain these abscesses.2
We have done this study to find out the prevalence of amoebic and PLA among patients of liver abscess. The clinical, personal, and demographical details were also evaluated to find out the risk factor associated with ALA and PLA, respectively.
Materials and Methods
This retrospective, single-center study was done in the bacteriology and parasitology section of the department of microbiology at a tertiary care center, where data of patients with liver abscess from January 2021 to December 2022 were collected from laboratory records and hospital information system.
Inclusion criteria: All patients whose liver abscess pus was drained therapeutically under USG guidance and was sent to the bacteriology section for pus culture and sensitivity were included in the study. Blood samples of these patients have also been received in the parasitology section on the same day for E. histolytica IgG testing. All these patients were diagnosed of liver abscess by USG findings.
Exclusion criteria: Patients of liver abscess whose aspirated pus was only sent for bacterial culture and no blood sample was sent to the parasitology lab were not included in the study.
A total of 107 samples of liver abscess pus were received in the bacteriology lab during this period whose blood samples were also received in parasitology lab. The study was approved by the Institutional Ethics Committee (IEC: 2021-109-IMP-EXP-38).
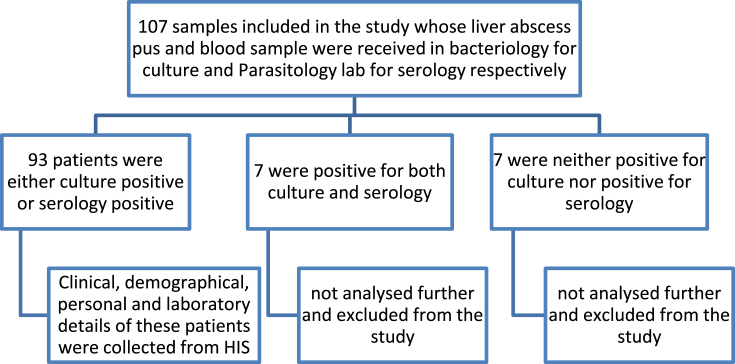
The methodology used in this study is illustrated in Figure 1. Direct Gram smear and pus culture and sensitivity were performed on the aspirated pus. Blood agar and MacConkey agar were used for the culture. The incubation conditions were aerobic incubation in 37 °C for MacConkey agar and CO2 incubator for blood agar for 18–24 h. Enzyme-linked immunosorbent assay (ELISA) for detection of E. histolytica IgG was done using a Novatech IgG ELISA kit. ELISA was performed, and results were interpreted according to manufacturer's guidelines.
Figure 1.
Detailed flowchart of the methodology used in the study.
The demographic details, personal history including addiction history, and clinical details were collected from hospital information system. Laboratory investigations including total leukocyte counts, platelets, bilirubin, erythrocyte sedimentation rate, hemoglobin, glycosylated hemoglobin, alkaline phosphatase, alanine transaminase, aspartate transaminase, serum albumin, bilirubin levels, and procalcitonin were recorded. The USG findings regarding the size, location, volume, and number of abscesses were also analyzed.
The data were compiled in an Excel sheet and were analyzed. The quantitative variables were analyzed using range and median. Statistical tests were performed using Statistical Package for the Social Sciences (IBM-SPSS) software (Version 20; Armonk, N.Y.) for descriptive statistics. The significance among percentages was calculated with the Chi-square test, and a P value of <0.05 was considered statistically significant.
Results
A total of 107 patients of liver abscess whose liver abscess pus and blood samples were received in bacteriology for culture and in parasitology lab for serology, respectively, were included in our study.
The age range of the population was 1–86 years (median = 45 years). The male-to-female ratio was 2.68:1. The maximum cases belonged to the age group of 41–60 years (n = 41).
Amongst the 107 cases of liver abscess, 66 (61.6%) were diagnosed to be ALA by positive E. histolytica IgG serology, 27 (25.23%) were diagnosed as PLA by positive pus cultures, 7 (6.5%) were positive for both E. histolytica IgG serology and pus culture, and 7 (6.5%) were neither positive for E. histolytica serology nor pus culture.
The objective of our study was to compare the demographic, personal, and clinical characteristics of patients with liver abscess having amoebic or pyogenic etiology. So, the demographic and personal history of the patients with ALA (n = 66) and PLA (n = 27) are given in Table 1.
Table 1.
The Demographic and Personal History of the Patients with Amoebic Liver Abscess and Pyogenic Liver Abscess.
| ALA (n = 66) (%) | PLA (n = 27) (%) | P-value | |
|---|---|---|---|
| Gender | |||
| Male | 49 (74.25) | 18 (66.67) | – |
| Female | 17 (25.75) | 9 (33.33) | |
| Age | |||
| 0–20 | 6 (9.09) | 6 (22.22) | – |
| 21–40 | 21 (31.81) | 5 (18.51) | |
| 41–60 | 26 (39.39) | 13 (48.14) | |
| 61–80 | 13 (19.69) | 1 (3.7) | |
| 81–100 | 0 | 2 (7.4) | |
| Addictions | |||
| Alcohol consumption | 38 (57.57) | 8 (29.62) | 0.01 |
| Tobacco chewers | 9 (13.63) | 1 (3.7) | 0.19 |
| Cigarette smokers | 2 (3.03) | 1 (3.7) | 0.86 |
ALA: amoebic liver abscess; PLA: pyogenic liver abscess
The age range of patients of ALA and PLA were 8–79 years (median = 46 years) and 1–86 (median = 43 years), respectively. Male population of 41- to 60-years age group was prone to development of ALA as compared to PLA. Also, alcohol consumption was found to be a statistically significant (P-value: 0.016; odds ratio [OR]: 3.22; 95% confidence interval [CI]: 1.23 to 8.41) risk factor in case of ALA as compared to PLA. And tobacco chewing (P-value: 0.19; OR: 4.10; 95% CI: 0.49 to 34.11) and cigarette smoking (P-value: 0.86; OR: 0.81; 95% CI: 0.07 to 9.35) were not found to be a statistically significant risk factors in both these groups (Table 1).
Majority of the patients of ALA and PLA had symptoms that lasted for more than 1 week before which they came for hospitalization. The major complaints were right upper quadrant pain and fever in both the groups. Vomiting and breathlessness also were complaints found in ALA. These symptoms did not show any statistical association with PLA and ALA. Most of the patients had a hospital stay of 7–30 days (Table 2).
Table 2.
Major Presenting Symptoms Along with Their Duration and the Hospital Course of Patients of Pyogenic and Amoebic Liver Abscess.
| ALA (n = 66) (%) | PLA (n = 27) (%) | P-value | |
|---|---|---|---|
| Duration of symptoms | |||
| ≥7 days | 39 (59.1) | 25 (92.59) | 0.005 |
| <7 days | 27 (40.9) | 2 (7.41) | |
| Presenting symptoms | |||
| Right upper quadrant pain | 54 (81.81) | 23 (85.18) | 0.69 |
| Fever | 57 (86.36) | 24 (88.89) | 0.74 |
| Vomiting | 16 (24.24) | 4 (14.81) | 0.32 |
| Breathlessness | 16 (24.24) | 2 (7.4) | 0.07 |
| Diarrhea | 4 (6.06) | 1 (3.7) | 0.65 |
| Loss of appetite | 6 (9.09) | 1 (3.7) | 0.38 |
| Weakness | 1 (1.51) | 1 (3.7) | 0.52 |
| Duration of hospital stay | |||
| ≤7 days | 14 (21.21) | 6 (22.22) | – |
| 7–30 days | 48 (72.72) | 19 (70.37) | |
| >31 days | 4 (6.06) | 2 (7.4) | |
ALA: amoebic liver abscess; PLA: pyogenic liver abscess
From Table 3, it was observed that hepatomegaly was a major sign found in patients with ALA, and it was found to be significantly associated. Total leucocyte count (TLC) count was not found to be significantly increased in either of the groups, but platelet-to–white blood cell (WBC) (PWR) ratio average was 18.06 in PLA patients and 23.4 in ALA patients. Accordingly, numbers of patients who had PWR values less than average were 2 and 13 in ALA and PLA, respectively. Elevated ESR values were seen in patients of both ALA and PLA and did not show any significant association with any of these groups. Decreased hemoglobin was found to be significantly associated with patients of ALA. Diabetes was not found as a significant risk factor in patients with ALA as majority of the patients had normal glycosylated hemoglobin (HbA1c) values, but it was significant in patients with PLA. Elevated bilirubin was observed in patients with ALA as compared to patients with PLA but was statistically nonsignificant. Also, majority of the patients of PLA had elevated procalcitonin values. Elevated alkaline phosphate (ALP) and serum glutamic-oxaloacetic transaminase (SGOT) values with normal serum glutamic-pyruvic transaminase (SGPT) values was a pattern seen in patients of ALA, but none of them were statistically significant to ALA. Decreased serum albumin was a predominant and significant finding in patients with PLA (Table 3).
Table 3.
Major Clinical Signs and Laboratory Parameters of the Study Population.
| Normal range | ALA (n = 66) | PLA (n = 27) | P-value | |
|---|---|---|---|---|
| Hepatomegaly | >15 cm | 49 | 12 | 0.007 |
| Total leucocyte count | ||||
| Normal | 4000–11000 | 32 | 14 | 0.76 |
| Elevated | >11,000 | 34 | 13 | |
| Platelet-to–white blood cell ratio | Lower than average | 2 | 13 | <0.0001 |
| Increased ESR | >20 mm | 65 | 27 | 0.63 |
| Hemoglobin | ||||
| Normal | 11–15 | 22 | 16 | 0.02 |
| Decreased | <11 | 44 | 11 | |
| Glycosylated hemoglobin | ||||
| Normal | <5.7 | 43 | 8 | – |
| Pre-diabetic | 5.7–6.4 | 9 | 6 | |
| Diabetic | >6.5 | 14 | 13 | |
| Elevated bilirubin | >0.4 | 43 | 14 | 0.23 |
| Procalcitonin | ||||
| Normal | <0.1 | 4 | 1 | – |
| Bacterial infection unlikely | 0.1–0.25 | 9 | 3 | |
| Bacterial infection possible | 0.25–0.5 | 12 | 1 | |
| Presence of bacterial infection | >0.5 | 11 | 22 | |
| Alkaline phosphatase | ||||
| Normal | 44–147 | 20 | 7 | 0.67 |
| Elevated | >147 | 46 | 20 | |
| SGOT | ||||
| Normal | 5–45 | 26 | 18 | 0.584 |
| Elevated | >45 | 40 | 9 | |
| SGPT | ||||
| Normal | 5–45 | 42 | 18 | 0.781 |
| Elevated | >45 | 24 | 9 | |
| Albumin | ||||
| Normal | 3.8–5.5 | 53 | 5 | <0.0001 |
| Low | <3.7 | 13 | 22 | |
ALA: amoebic liver abscess; PLA: pyogenic liver abscess; SGOT: serum glutamic-oxaloacetic transaminase; SGPT: serum glutamic-pyruvic transaminase; ESR:
A single abscess in the right lobe of liver involving the 6th and 7th segments was characteristic of liver abscess with amoebic etiology, whereas multiple abscesses involving the right lobe of liver was characteristic of PLA. ALA was more prone to rupture (15.1%) (Table 4).
Table 4.
Ultrasound Investigation Findings Regarding the Characteristic Features of the Abscess in Amoebic and Pyogenic Liver Abscess Patients.
| ALA (n = 66) (%) | PLA (n = 27) (%) | |
|---|---|---|
| Number of abscess | ||
| >1 | 16 (24.24) | 19 (70.37) |
| 1 | 50 (75.75) | 8 (29.62) |
| Volume of abscess | ||
| <50 ml | 15 (22.72) | 2 (7.4) |
| 50–100 ml | 14 (21.21) | 11 (40.74) |
| 100–200 ml | 19 (28.78) | 6 (22.22) |
| 200–500 ml | 12 (18.18) | 5 (18.51) |
| 500–1500 ml | 6 (9.09) | 3 (11.11) |
| Lobes involved | ||
| Right lobe only | 53 (80.3) | 22 (81.48) |
| Left lobe only | 7 (10.61) | 3 (11.11) |
| Right and left lobes | 5 (7.57) | 2 (7.4) |
| Caudate lobe | 1 (1.51) | 0 |
| Segments involved | ||
| 6th and 7th segments | 35 (53.03) | 14 (51.85) |
| 8th segment | 19 (28.79) | 5 (18.51) |
| Other segments | 12 (18.18) | 7 (25.92) |
| Ruptured abscess | 10 (15.15) | 2 (7.4) |
ALA: amoebic liver abscess; PLA: pyogenic liver abscess
Among the causes of PLA, the most common causative bacteria was E. coli (n = 8), followed by E. cloacae (n = 5) and Citrobacter freundii (n = 4). Two cases each were reported of Acinetobacter lwoffii, Staphylococcus aureus, and Pseudomonas species. Acinetobacter baumannii, Klebsiella pneumoniae, Streptococcus spp., and Coagulase-negative Staphylococcus were reported in only 1 patient, respectively.
Mortality was seen in 6 (6.4%) patients, among which, 5 had ALA, and 1 patient had PLA.
Discussion
Among the 107 cases of liver abscess, 66 (61.6%) were diagnosed to be ALA by positive E. histolytica IgG serology, and 27 (25.23%) cases were diagnosed as PLA by positive pus cultures. Similar findings were reported in a study by Jaiswal et al., in which, out of the 200 patients of liver abscess, 20.5% were identified as PLA, and 68% were classified as ALA.14 A study from Kerala also documented similar findings, where among 50 cases of liver abscess, 68% and 22% were due to amoebic and pyogenic etiologies, respectively.15 A study by Ghosh S also reported that out of 200 cases of liver abscess, the prevalence of ALA and PLA were 69% and 18%, respectively.6 Also, in a study in Pakistan, they observed that amongst 577 cases, 82% were due to amoebic etiology, and 18% were due to pyogenic etiology.2
In case of ALA, males of 20–60 years of age were predominantly affected with a single abscess in the right lobe of liver. Similar results were observed in other studies also.16,17 Lodhi S reported that males were more affected than females in ALA.2 In a study by Mukhopadhyay M, it was found that ALA was most commonly seen in the population of age group 30–40 years.18 The right lobe was found to be the most affected lobe with solitary lesions. This is supported by findings in other studies where 70% of the abscesses were in the right lobe, and 77% were solitary abscesses.13 Ghosh S also reported that the right lobe was involved in amoebic and pyogenic liver abscesses.6 As observed in the present study, multiple abscesses were associated with PLA, which was also seen in a study by Kurland JE.17
Fever and right upper quadrant pain were the most common presentations of the patients in our study. Similar findings could be seen in literature also. Wang noted that patients of both groups presented with fever, pain in abdomen, and chills.16 A similar triad was observed by Lodhi S in patients of ALA and by Serranio C in PLA patients.2,19
It could also be seen that alcohol consumption was found to be a statistically significant (P-value: 0.016; OR: 3.22; 95% CI: 1.23 to 8.41) risk factor in case of ALA as compared to PLA. Various studies have been documented which suggest 35–87% patients of ALA to be associated with alcohol consumption.4,18,20, 21, 22, 23
Anemia in patients with ALA (66.67%) was also a predominant finding in our study. Chethan L also observed that 63% of the ALA patients had decreased hemoglobin.4 Another striking feature that we observed in our study was that the TLC count was neither significant in ALA nor PLA patients, although many studies have noted increased TLC count in PLA patients,16 but there are evidences in literature that also support the fact that an elevated TLC was significant in none of the two groups (ALA: 19.1%; PLA: 18.9%).2 This indicates that TLC cannot be considered as a relevant parameter in patients of liver abscess.
PWR was found to be a feasible marker for PLA prognosis in a study by Dong Gyun Ko. In our study, the PWR was low in majority of PLA patients as compared to ALA patients. This is in concordance with findings of Dong Gyun Ko, where he found that a low PWR was seen in PLA patients and that it can lead to complications.24
We found that diabetes was found as a significant risk factor in PLA patients (19/27; 70.37%) as compared to ALA patients (23/66; 34.8%). This was in agreement with findings by Wang J et al., where he found 81.5% of the patients with PLA to be associated with diabetes.16 Chang Jae Lee also found similar findings in his study, where he found higher proportion of elevated HbA1c values among patients of PLA than in ALA patients.25 Also, Lodhi S also reported that patients of PLA had elevated HbA1c values.2
Elevated bilirubin among ALA patients (43/66; 65%) was reported in our study. There are evidences in literature to support this finding. A study by Anil Kumar S reported that hyperbilirubinemia was associated with ALA.26
In our study, a pattern of increased ALP and SGOT with normal SGPT values and decreased albumin levels was seen in patients of ALA, but contradictory to that, some studies have reported a pattern of elevated ALP, SGOT, and SGPT values with decreased albumin in patients of ALA.22 Though the pattern was not observed in our study, individual variables were found to have significant correlation. Low serum albumin was found to be a statistically significant risk factor for PLA. This is in concordance with other studies.2,13,27 There are some studies that suggest correlation of hypoalbuminemia with ALA, 28,29 but in a study by Lal SB et al., the authors documented that hypoalbuminemia was associated with complicated liver abscess.30,31 In our study, we have just tried to identify the pattern in our institution.
The rupture of ALA is a major concern as it might cause complications. In our study, 10 of 66 (15.15%) of the ALAs were ruptured at the time of presentation. Among these, 4 were diagnosed of pleural effusion, 3 had ascites, and 3 had signs of sepsis. A study by Mukhopadhyay M also reported that 19 of 72 cases of ALA had complications due to abscess rupture and they were jaundice, ascites, subhepatic effusion, and intrapleural rupture.18
In our study, the most common bacterial cause of PLA was E. coli, followed by E. cloacae. Similar findings were seen in other studies where E. coli was the predominant cause of PLA.14,19,32
Overall mortality was seen in 6 (6.4%) patients among which 5 had ALA and one patient had PLA. In the present study, mortality rate was 9.09% and 3.70% in ALA and PLA patients, respectively. The mortality rate in ALA patients ranges from 2% to 15%.10 In a study from Chandigarh, the mortality rate among patients of ALA was found to be 5.8%.11 In a study by Czerwonko ME et al., mortality among PLA patients was found to be 7.8%.12 The high percentage of mortality among the ALA and PLA patients might be because our institution is a referral center and most of the patients admitted here are critical patients.
Limitation
Due to the small sample size, the prevalence found for ALA and PLA are corresponding to the tertiary care center and are not representative of the geographical area. Also, since it was a retrospective study, only cases that were reorted during that period were enrolled; therefore, prevalence could not be indicative of the prevalence in that area. Also, the treatment given to the patients and their antimicrobial susceptibility have not been discussed in this study. Though a high mortality rate has been documented in our study, it cannot be considered as conclusive of mortality burden among patients with liver abscess as our institution is a referral center.
Liver abscess is found to have relatively high mortality and morbidity in case of associated risk factors and deranged laboratory parameters. Therefore, early diagnosis is the only method to prevent mortality and morbidity in these patients. Since the presentation is very nonspecific, evaluation of certain risk factors and laboratory parameters can aid in the diagnosis.
credit authprship contribution statement
Deepika Sarawat - Study concept and design formulation, Writing the draft.
Gerlin Varghese - Helped in formulating the methodology, Analysis and interpretation of data.
Chinmoy Sahu - Editing of the draft.
Nidhi Tejan – Revising the draft critically for important intellectual content.
Surender Singh – Helped with the clinical data acquisition and the interpretation.
Sangram Singh Patel - Final approval of the version to be published.
Mohd Rashid Khan - Acquisition of data.
Conflicts of interest
The authors have none to declare.
Acknowledgement
I would like to thank the staff of Department of Microbiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences for the support and help.
Funding
None.
References
- 1.Lardière-Deguelte S., Ragot E., Amroun K., et al. Hepatic abscess: diagnosis and management. J Vis Surg. 2015;152:231–243. doi: 10.1016/j.jviscsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Lodhi S., Sarwari A.R., Muzammil M., Salam A., Smego R.A. Features distinguishing amoebic from pyogenic liver abscess: a review of 577 adult cases. Trop Med Int Health. 2004;9:718–723. doi: 10.1111/j.1365-3156.2004.01246.x. [DOI] [PubMed] [Google Scholar]
- 3.Arellano-Aguilar G., Marín-Santillán E., Castilla-Barajas J.A., Bribiesca-Juárez M.C., Domínguez-Carrillo L.G. A brief history of amoebic liver abscess with an illustrative case. Rev Gastroenterol México. 2017;82:344–348. doi: 10.1016/j.rgmx.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Chethan L. Clinical study of liver abscess. Int J Surg. 2021;8:2558–2565. doi: 10.18203/2349-2902.isj20213191. [DOI] [Google Scholar]
- 5.Ghosh P.K., Mandal N., Majhi J., Nandi M.M., Kuiri S.S., Ghosh G. Pyogenic liver abscess: prospective evaluation of USG guided percutaneous therapeutic aspiration. IOSR J Dent Med Sci. 2014;13:9–15. [Google Scholar]
- 6.Ghosh S., Sharma S., Gadpayle A.K., et al. Clinical, laboratory, and management profile in patients of liver abscess from northern India. J Trop Med. 2014;2014 doi: 10.1155/2014/142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fotedar R., Stark D., Beebe N., Marriott D., Ellis J., Harkness J. PCR detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii in stool samples from Sydney, Australia. J Clin Microbiol. 2007;45:1035–1037. doi: 10.1128/JCM.02144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zengzhu G., Bracha R., Nuchamowitz Y., Cheng -I.W., Mirelman D. Analysis by enzyme-linked immunosorbent assay and PCR of human liver abscess aspirates from patients in China for Entamoeba histolytica. J Clin Microbiol. 1999;37:3034–3036. doi: 10.1128/JCM.37.9.3034-3036.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caballero-Salcedo A., Viveros-Rogel M., Salvatierra B., et al. Seroepidemiology of amebiasis in Mexico. Am J Trop Med Hyg. 1994;50:412–419. doi: 10.4269/ajtmh.1994.50.412. [DOI] [PubMed] [Google Scholar]
- 10.Stanley S.L., Jr. Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 11.Sharma N., Sharma A., Varma S., Lal A., Singh V. Amoebic liver abscess in the medical emergency of a North Indian hospital. BMC Res Notes. 2010;3:21. doi: 10.1186/1756-0500-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czerwonko M.E., Huespe P., Bertone S., et al. Pyogenic liver abscess: current status and predictive factors for recurrence and mortality of first episodes. HPB (Oxford) 2016;18:1023–1030. doi: 10.1016/j.hpb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimian J., Wilson T., Oram V., Holzman R.S. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 14.Jaiswal V., Ghoshal U., Baijal S.S., Mittal B., Dhole T.N., Ghoshal U.C. Evaluation of antigen detection and polymerase chain reaction for diagnosis of amoebic liver abscess in patients on anti-amoebic treatment. BMC Res Notes. 2012;5:416. doi: 10.1186/1756-0500-5-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon A.R., Kizhakkekarammal P.K., Rao G.K. Amoebic vs pyogenic liver abscesses: a comparative study in a tertiary care hospital. J Acad Clin Microbiol. 2015;17:89. [Google Scholar]
- 16.Wang W.J., Tao Z., Wu H.L. Etiology and clinical manifestations of bacterial liver abscess: a study of 102 cases. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000012326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurland J.E., Brann O.S. Pyogenic and amebic liver abscesses. Curr Gastroenterol Rep. 2004;6:273–279. doi: 10.1007/s11894-004-0078-2. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay M., Saha A.K., Sarkar A., Mukherjee S. Amoebic liver abscess: presentation and complications. Indian J Surg. 2010;72:37–41. doi: 10.1007/s12262-010-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serraino C., Elia C., Bracco C., et al. Characteristics and management of pyogenic liver abscess: a European experience. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jindal A., Pandey A., Sharma M.K., et al. Management practices and predictors of outcome of liver abscess in adults: a series of 1630 patients from a liver unit. J Clin Exp Hepatol. 2021;11:312–320. doi: 10.1016/j.jceh.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhary S., Noor M.T., Jain S., Kumar R., Thakur B.S. Amoebic liver abscess: a report from central India. Trop Doct. 2016;46:12–15. doi: 10.1177/0049475515592283. [DOI] [PubMed] [Google Scholar]
- 22.Manjavkar S. Correlation between abscess size and liver function tests in cases of liver abscess. Int J Res Med Sci. 2017;5:3340. [Google Scholar]
- 23.Rakesh M., Debabrata B., Debarshi J. Clinical profile and management of patients of amoebic liver abscess in a tertiary care centre. Int J Sci Res. 2020:5–7. doi: 10.36106/ijsr/5406508. [DOI] [Google Scholar]
- 24.Ko D.G., Park J.W., Kim J.H., et al. Platelet-to-White blood cell ratio: a feasible biomarker for pyogenic liver abscess. Diagnostics. 2022;12:2556. doi: 10.3390/diagnostics12102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C.J., Han S.Y., Lee S.W., et al. Clinical features of gas-forming liver abscesses: comparison between diabetic and nondiabetic patients. Korean J Hepatol. 2010;16:131–138. doi: 10.3350/kjhep.2010.16.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Anil Kumar S., Mishra A., Malhotra N., Alpana M. Hyperbilirubinemia in patients with amoebic liver abscess: a study of 75 cases. J Gastro & Digestive Systems. 2013;3 [Google Scholar]
- 27.Barnes P.F., DeCock K.M., Reynolds T.N., Ralls P.W. A comparison of amebic and pyogenic abscess of the liver. Medicine (Baltim) 1987;66:472–483. doi: 10.1097/00005792-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sharma M.P., Dasarathy S., Verma N., Saksena S., Shukla D.K. Prognostic markers in amebic liver abscess: a prospective study. Am J Gastroenterol. 1996;91:2584–2588. [PubMed] [Google Scholar]
- 29.Sánchez-Aguilar M, Morán-Mendoza O, Herrera-Hernández MF, Hernández-Sierra JF, Mandeville PB, Tapia-Pérez JH et al. Prognostic indications of the failure to treat amoebic liver abscesses. Pathog Glob Health. Aug; 106:232-237. doi: 10.1179/2047773212Y.0000000021. [DOI] [PMC free article] [PubMed]
- 30.Lal S.B., Venkatesh V., Kumar A., et al. Liver abscess in children-experience from a single tertiary care center of north India: etiology, clinical profile and predictors of complications. Pediatr Infect Dis J. 2021;40:e179–e184. doi: 10.1097/INF.0000000000003053. [DOI] [PubMed] [Google Scholar]
- 31.Jha A.K., Jha P., Chaudhary M., et al. Evaluation of factors associated with complications in amoebic liver abscess in a predominantly toddy-drinking population: a retrospective study of 198 cases. JGH Open. 2019;3:474–479. doi: 10.1002/jgh3.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayek I., Onat D. InSurgical Treatment: Evidence-Based and Problem-Oriented. Zuckschwerdt; 2001. Pyogenic and Amebic Liver Abscess. [Google Scholar]