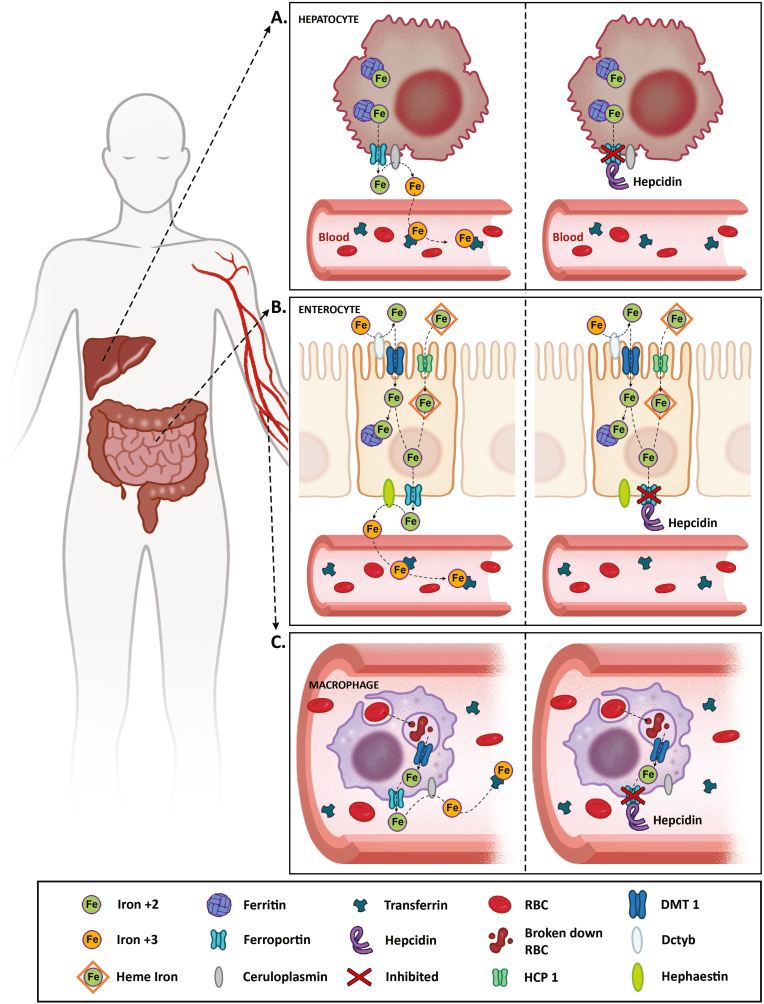
Fig. 1.
Iron absorption and recycling. Hepcidin plays a critical role in regulating systemic iron availability and enteral iron absorption by modulating FPN levels. During iron deficiency, stored iron in hepatocytes (A) is exported to the systemic circulation by FPN. Here ferrous iron is oxidized by ceruloplasmin and bound to transferrin for further transport and utilization. In enterocytes (B), non-heme iron is reduced from ferric to ferrous iron by Dcytb at the apical border. Ferrous iron is taken up into the enterocyte by DMT1, where it is stored bound to ferritin or exported by FPN to the systemic circulation, where it gets oxidized by hephaestin and bound to transferrin. Heme-iron uptake by enterocytes is not entirely understood, but it is believed to be absorbed by HCP1 and degraded by HO1. The released iron is bound to ferritin or exported into the systemic circulation, as necessary. Finally, senescent RBCs are phagocytosed by macrophages (C). RBCs are degraded in the lysosome, and heme is exported to the cytosol via HCP1. In the cytosol, iron is extracted by HO1 and stored as ferritin or exported to the systemic circulation by FPN. In an iron-replete state or cases of elevated hepcidin, hepcidin blocks, internalizes and degrades FPN. This causes iron restriction within the cells and prevents iron export to the circulation. DMT1: divalent metal transporter 1, Dcytb: duodenal cytochrome B, FPN: ferroportin, Fe3+: ferrous iron, Fe2+: ferric iron, HO1: heme oxygenase 1, HCP1: heme carrier protein 1, RBC: red blood cell. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)