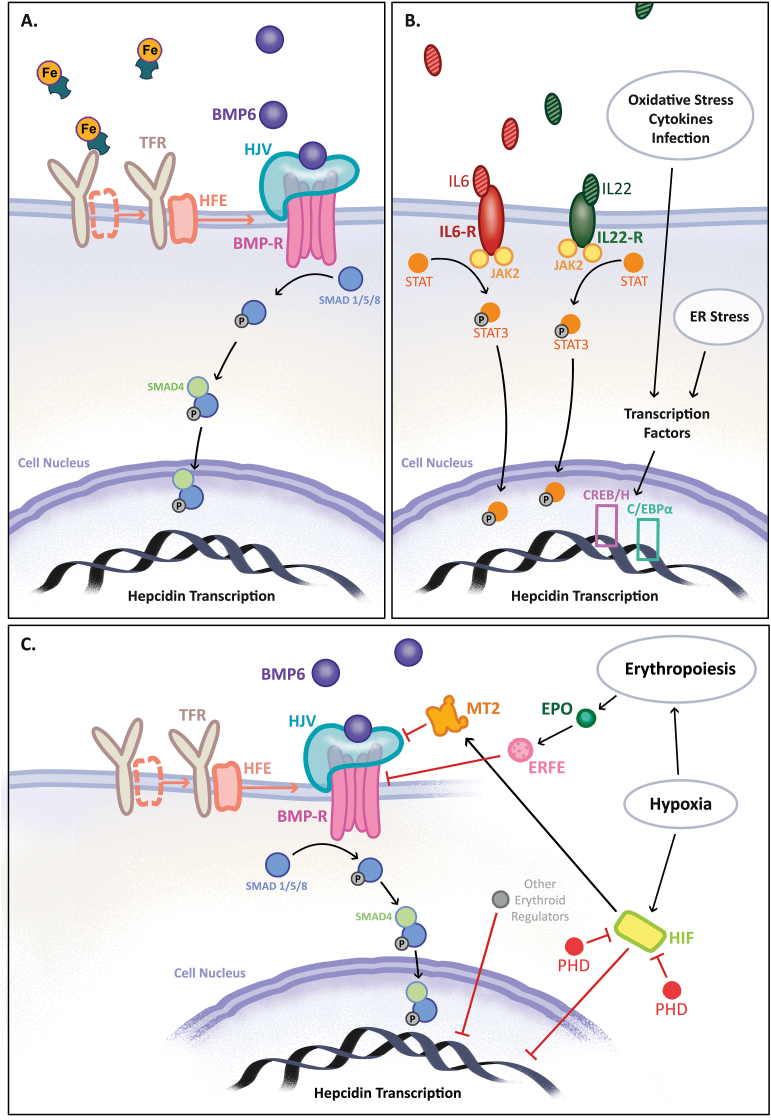
Fig. 2.
Regulation of hepcidin. (A) During iron overload, Tf-Fe attaches to TFR1, causing HFE to dissociate from TFR1 and bind to TFR2. The TFR-HFE complex binds to HJV, which is important for further signaling by BMP6. Elevated BMP6 levels during iron excess lead to its binding to the BMP-R and co-receptor HJV, triggering SMAD1/5/8 phosphorylation and binding to SMAD4. This complex then enters the cell nucleus and induces HAMP transcription. (B) During inflammation, IL-6 and IL-22 bind to their receptors, which activates JAK2 and leads to STAT3 phosphorylation. Phosphorylated STAT3 translocates to the cell nucleus and upregulates hepcidin by inducing transcription of the HAMP gene. In addition, other cytokines, oxidative stress, ER stress, and infectious inflammation upregulate hepcidin by inducing transcription factors, such as the CREBH or C/EBPα. (C) Iron deficiency, hypoxia, and ineffective erythropoiesis can downregulate hepcidin through multiple pathways that are not entirely understood. Under normoxic conditions, PHDs inhibit HIFs. During hypoxia, HIFs upregulate protease MT2, which inhibits BMP-SMAD signaling and decreases hepcidin expression. Hypoxia can also directly downregulate HAMP transcription. During increased erythropoiesis, EPO upregulates ERFE, which is known to inhibit BMP-SMAD signaling. Other potential erythroid regulators, such as GDF15, might also downregulate hepcidin. BMP6: bone morphogenetic protein 6, BMP-R: BMP receptor, EPO: erythropoietin, ERFE: erythroferrone, ER: endoplasmic reticulum, GDF15: growth and differentiation factor 15, HFE: human hemochromatosis protein, HJV: hemojuvelin, HIF: hypoxia-inducible transcription factor, IL: interleukin, JAK2: Janus kinase 2, MT2: matriptase 2, PHD: prolyl hydroxylase, SMAD: mothers against decapentaplegic, STAT3: signal transducer and activator of transcription-3, Tf-Fe: iron bound to transferrin, TFR: transferrin receptor.