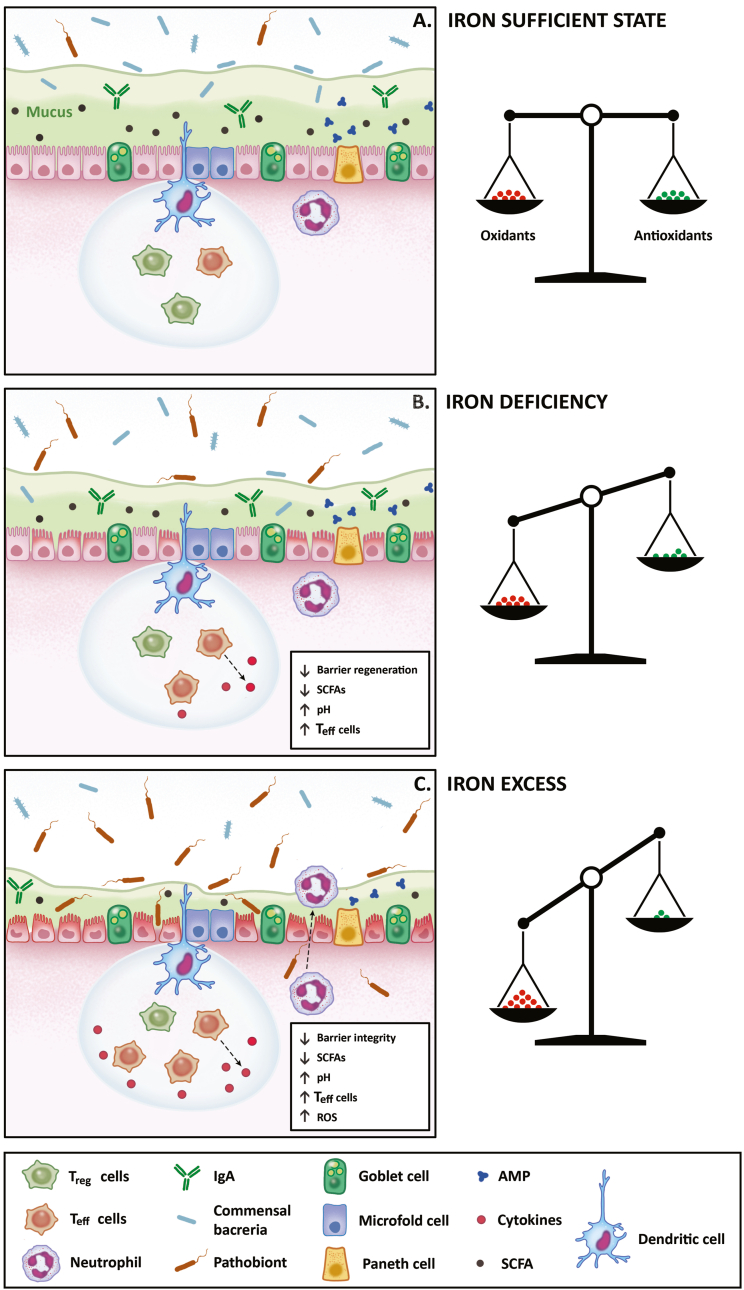
Fig. 4.
High-level overview of changes in redox status and the intestinal microbiota during iron deficiency and excess. (A) Under physiological conditions, the intestines are lined by a single layer of intestinal cells that do not come into direct contact with bacteria. In order to keep this physiological homeostasis, the intestines utilize several defense mechanisms. Firstly, enterocytes and goblet cells produce mucus that makes a thick inner layer, which is almost impenetrable to the bacteria, and an outer layer, which is less thick and can contain commensal bacteria. Secondly, Paneth cells and enterocytes secrete antimicrobial proteins and immunoglobulins into the mucus layer. Thirdly, the dendritic and microfold cells sample bacterial antigens and induce inflammatory cascades directed against these pathogens, which involves regulatory and effector T cells and pro- or anti-inflammatory cytokines. Finally, the intestines contain neutrophils which can trap and eliminate the bacteria in an oxidative burst. Under normal physiologic conditions, there is a balance between antioxidants and prooxidants, which prevents tissue and cell damage. (B) During iron deficiency, antioxidant activity is reduced and might result in oxidative stress. In the intestines, bacteria compete for iron leading to decreases in SCFA-producing bacteria, which increases luminal pH and pathobiont virulence, and results in decreased intestinal barrier regeneration. In addition, SCFAs modulate immune responses via the NF-kβ pathway, which ensures the balance between regulatory and effector T cells and anti-inflammatory cytokine production. However, during iron deficiency, the lack of SCFAs leads to increased production of effector T cells and pro-inflammatory cytokines. (C) During iron excess, there is an imbalance between antioxidant availability and the production of reactive species, resulting in oxidative stress. In the intestines, fewer SCFA-producing bacteria might result in changes similar to those during iron deficiency. However, pathogenic bacteria increase in abundance and virulence during iron excess and can damage the epithelium. In addition, oxidative burst by neutrophils in response to bacteria creates more reactive oxygen and nitrogen species, which further increases the damage to the intestinal epithelium. AMP: antimicrobial peptides, IgA: immunoglobulins, ROS: reactive oxygen species, SCFAs: short-chain fatty acids, Teff: T effector cells, Treg: T regulatory cells.