Abstract
The processes of microbiological destruction of toxic and large-tonnage waste are the most attractive processes for protecting the environment. The review considers the results of studies of microbial decomposition of nitrate esters, including hardly decomposable nitrocellulose. The published data show that specific microorganisms are able to degrade nitrated cellulose compounds under both anaerobic and aerobic conditions. The most promising microorganisms in terms of the efficiency of the nitrocellulose degradation process are bacteria belonging to Desulfovibrio genera, fungi Fusarium solani and Sclerotium rolfsii, as well as their co-cultivation. Recently, the first information about the enzymes involved in the process of nitrocellulose degradation, possible mechanisms of reactions carried out by these enzymes, and the effect of electron donors and acceptors adding to the process have been obtained. Contamination of industrial wastewater with nitrocellulose leads to treatment necessity by using cost-effective, harmless methods. A combined aerobic-anaerobic system, including both bacteria and fungi, has shown hopeful results.
Keywords: Nitrocellulose, Microbial degradation, Bacteria, Fungi, Desulfovibrio desulfuricans, Fusarium solani
Introduction
The nitrocellulose (NC) is an ester of cellulose and nitric acid with chemical composition [C6H7O2(OH)3−x(ONO2)x]n, where x denotes the degree of substitution and defines the nitrogen content for a given NC material. NC is a necessary material providing modern scientific and technological progress and is widely applied in defense, chemical, and other industries for the production of fuel of diverse types, vanishes, paints, etrols, etc. (Sullivan et al. 2020). NC with a nitrogen content of less than 12% is exploited as collodions, while higher-nitrated NC is highly explosive and used in the military industry (Liu 2019). These productions form large amounts of effluents containing nitrocellulose fiber.
As recent research has shown, the wastewater from NC production is toxic to microorganisms (Ribeiro et al. 2013); moreover, it can form sludge with explosive characteristics.
The structural features of the NC make it persistent to microbial destruction under natural conditions (Auer et al. 2005; Giacomucci et al. 2012; Petrova et al. 2002; Saratovskikh et al. 2018a; Saratovskikh et al. 2018b). Resistance to biological degradation results in the accumulation of wastes as bottom deposits in pool settlings or in slurry tanks from which they can get into water basins. They can contain thousands of tons of fibrous polymer, which exerts a negative effect on the environment and human health.
The presently applied methods of waste processing in NC productions have a number of substantial drawbacks. Such physicochemical methods as membrane filtration using coagulating agents, chemical hydrolysis, photodegradation, and combustion are expensive and environmentally unsafe (Berthumeyrie et al. 2014; El-Diwani et al. 2009; Pouretedal et al. 2021; Ugurlu and Ozturkcu 2018; Wang et al. 1982). Since biochemical processes caused by random microflora are unpredictable, waste composting and disposal give no exact prediction of the results of its enzymatic activity toward NC itself and its degradation products (White and Snape 1993). Introducing deterrents improves the thermal stability of NC and complicates the situation (Li et al. 2022).
An ideal economic activity of humans should be constructed according to the principle of natural ecosystems, which optimally consume matter and energy and in which wastes of some organisms serve as a biotope for other organisms; i.e., circulation (or waterless utilization technologies) takes place. The corresponding system of decontamination of the industrial chemical industry is possible only when using biological oxidation methods. The development of methods for the utilization of toxic wastes from typical NC productions under artificial conditions would possibly solve environmental problems, diminish a negative load on the natural environment, and become a basis for the solution of an acute economic task.
The present review generalizes the results of searching for efficient microorganisms which can be used as destructors in the biological treatment of effluents of NC production and compares them with available literature data.
Nitroesterase activity in microorganisms
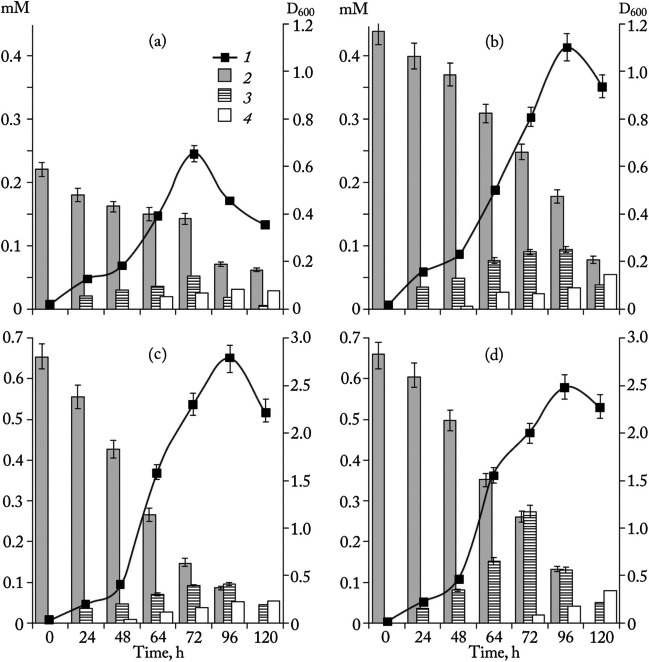
Various taxonomic groups of pro- and eukaryotes have been shown to possess enzymatic activity hydrolyzing bonds of nitro groups in the xenobiotic compounds. These studies were begun mainly on the example of trinitrotoluene (TNT) in the 1980s of the last century, and now the results of the transformation and mineralization of TNT under both aerobic and anaerobic conditions have been obtained. Biodegradability of TNT has been reported within bacteria of genera Achromobacter, Bacillus, Citrobacter, Klebsiella, Mycobacterium, Nocardia, Pseudomonas, Rhodococcus, and Stenotrophomonas as well as fungi genera Yarrowia, Irpex, and Phanerochaete (Anasonye et al. 2015; Gupta et al. 2023; Habineza et al. 2016; Jaafaryneya et al. 2023; Jain et al. 2004; Kim et al. 2002; Kim and Song 2003; Serrano-González et al. 2018). So, for example, some strains of gram-positive and gram-negative bacteria capable of decomposing TNT at comparatively high concentrations (up to 200 mg/L) were isolated (Zaripov et al. 2004). As can be seen from Fig. 1, the treatments with these bacteria led to the formation of hydroxylaminodinitrotoluenes, which demonstrated high biostability. The total decrease in the content of nitro groups does not exceed 10% of the initial concentration of TNT and was 0–3% in most cases. 3-Monoaminodinitrotoluenes were the next identified reaction products.
Fig. 1.
Dynamics of TNT decomposition on the growing culture of gram-positive bacteria (a) Bacillus sp. ZS19 and (b) Sarcina sp. IC2 and gram-negative bacteria (c) ZS180 and (d) ZS20: growth of bacteria (1) and changes in concentrations of TNT (2), hydroxylaminodinitrotoluenes (3), and monoaminodinitrotoluenes (4) (Zaripov et al. 2004)
According to published data (French et al. 1998; Haïdour and Ramos 1996; Spanggord et al. 1991; Vorbeck et al. 1998), the most part of aerobic bacteria reduces the nitro derivatives of monoamine compounds. Microbiological reduction of the ONO2 groups of NC can be the key reaction, decreasing toxicity and providing possibilities for its further deep transformations. The decomposition of TNT under the action of oxidoreductase enzyme was proposed earlier (Pak et al. 2000) and is shown in Fig. 2.
Fig. 2.
Transformation of TNT catalyzed by oxidoreductase (XenB) and proposed subsequent non-enzymatic reactions leading to the elimination of nitrite and formation of aminodimethyltetranitrobiphenyl (Pak et al. 2000)
The toxic effect of TNT toward bacteria is exhibited as the suppression of the culture growth and also as a change in the morphophysiological and physical properties of the cells (Kurinenko et al. 2007). At high TNT concentrations, the cell sizes decreased, granularity increased, and the rates of glucose utilization and breathing decreased. The Ca/K ratio in the cells contacting with TNT increased by 3.8 times compared to the cells that do not contact with xenobiotic substances. This confirms that in the presence of TNT cells, E. coli K12 existed at the initial stage of the hypometabolic state. The introduction of TNT changed the contents of both macro- and microelements in cells of E. coli K12. This change in the elemental composition of the cell composition was related to a change in transporting these substances, namely, to the violation of the structure of the external lipoproteid membrane. It is most likely that this occurred because of the violation of the protein–lipid interaction in the external lipoproteid membrane of cells of E. coli K12, which, in turn, resulted and the distortion of its transport properties, a change in ionic homeostasis, and transition of the bacteria to the hypometabolic state.
Degradation of nitrocellulose by sulfate-reducing bacteria
A study of the transformation of nitrocellulose under methanogenic conditions showed a decrease in the nitrogen content by at least 24%. Thus, biotransformed NC under anaerobic conditions had nitrogen content comparable to non-explosive nitrocellulose. This suggests that anaerobic treatment may be a technically feasible process for neutralizing NC (Freedman et al. 1996).
Effluents from the NC production contain significant amounts of sulfates, and hence, sulfate-reducing bacteria (SRB) were chosen as possible agents for NC decomposition. SRB is a specialized group of anaerobic bacteria that use sulfate as the terminal electron acceptor and are permanently present in very different natural and anthropogenic environments including soil, freshwater, marine water, soda and salt lakes, and waste treatment plant. Some representatives of the Desulfovibrio genus can use nitrates as electron acceptors (Cadby et al. 2017; Coelho and Romao 2015). Periplasmic nitrate reductase (NapA) in D. desulfuricans is unique, which contains iron-sulfur protein as a direct electron donor (Fig. 3) instead of c-type cytochrome (Marietou et al. 2005).
Fig. 3.
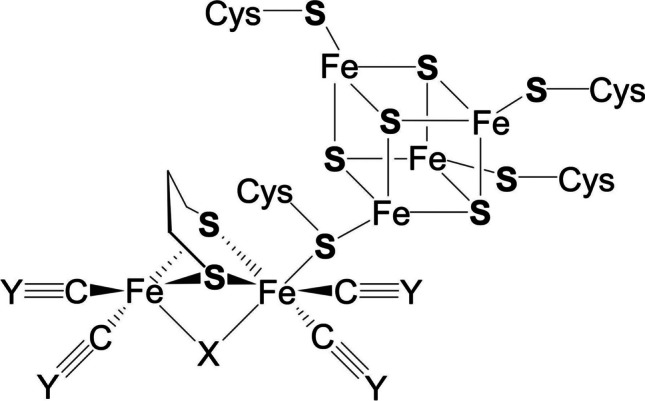
Prosthetic group of iron hydrogenase from Desulfovibrio desulfuricans. Cys is cysteine (α-amino-β-thiopropionic acid, 2-amino-3-mercaptopropanic acid HSCH2CH(NH2)COOH, aliphatic sulfur-containing amino acid)
The application of SRB as the primary unit in a microbial consortium for the initiation of NC decomposition under the conditions of industrial effluents was studied (Giacomucci et al. 2012; Petrova et al. 2002). D. desulfuricans were shown to decrease the degree of NC nitration by 6.7–7.8% and make it accessible for other members of the consortium. NC degradation study under sulfidogenic conditions by enrichment cultures showed that the growth medium should additionally contain an electron donor (methanol, lactate) to perform the biotransformation of NC (Freedman et al. 2002). In this case, the nitrogen content in the NC samples decreases from 13.2 to 12.3%. While pure cultures D. desulfuricans, D. vulgaris, and D. gigas were able to degrade NC by 4.9–9.3%. An appreciable decrease in the NC amount occurs in the stationary phase of the bacterial growth on the 8th day of cultivation. The dynamics of accumulation and consumption of free nitrate ions and ammonium ions indicate the transformation of NC and the use of products of this transformation by the bacterium D. desulfuricans itself. However, NC retarded these processes already from the first hours of cultivation, as it was shown by the study of the dynamics of lactate consumption and hydrogen sulfide formation by SRB in the presence of NC (Fig. 4).
Fig. 4.

Growth of bacteria Desulfovibrio desulfuricans (A) and consumption of lactate (B) (1) in control medium and (2) in the presence of NC (Petrova et al. 2002)
The retardation of the bacterial growth was expressed as some increase in the duration of the lag phase and a decrease in the specific growth rate and yield of the bacterial mass. A decrease in the molar economic coefficient indicated an increase in the consumption of the substrate for the synthesis of a biomass unit. An enhanced heat released in the presence of NC compared to the control can indicate that NC induced a disbalance of catabolic and anabolic processes in growing D. desulfuricans.
The depression of metabolic processes for D. desulfuricans in the presence of non-toxic compound NC can be related to free nitrate ions that got into the growth medium. The ability to dissimilation reduction of nitrates to ammonia was shown for SRB. With the simultaneous content of sulfates and nitrates in the medium, some SRBs reduce nitrates primarily, while others reduce sulfates (Dalsgaard and Bak 1994).
Although SRBs are obligate anaerobes, studies have shown that bacteria of the genus Desulfovibrio were tolerant to aerobic conditions (Fournier et al. 2004; Lobo et al. 2007). Thus, the activity of several key enzymes was analyzed according to different oxygen contents. The results showed that the activity of nitrate and nitrite reductase of D. desulfuricans did not substantially change in the presence of oxygen. The activity of [Fe]-, [NiFe]-, and [NiFeSe]-bound hydrogenases from the fractions of cells of D. desulfuricans grown under anaerobic conditions and in the presence of 18% O2 were also independent of the oxygen effect. The activities of catalase and dismutase superoxide increased due to the action of oxygen, whereas the activity of peroxidase remains unchanged (Table 1). The exposure of the cells to oxygen exerted no substantial effect on differences in the activities of membrane-bound breathing enzymes, such as succinate:DCPIP oxidoreductase and NADH:ferricyanide oxidoreductase, and consumption of oxygen by extracts of the membranes.
Table 1.
Comparison of the activities of enzymes from the cells of D. desulfuricans grown under anaerobic and aerobic conditions (Fournier et al. 2004)
| Activity | O2, 0% | O2, 18% |
|---|---|---|
| Nitrate reductase (nmol/min·mg) | 8 | 5 |
| Nitrite reductase (μmol/ min·mg) | 46 | 49 |
| Superoxide dismutase (U/mg) | 49 | 73 |
| Catalase (μmol/min·mg) | 78 | 142 |
| Peroxidase (L mol/min·mg) | 3 | 3 |
| NADH:Ferricyanide oxidoreductase (μmol/min·mg) | 8 | 7 |
| Succinate:DCPIP oxidoreductase (nmol/min·mg) | 59 | 61 |
| TMPD:oxygen oxidoreductase (nmol/min·mg) | 7 | 9 |
| Inhibition of O2 consumption (%) | 40 | 55 |
The property of bacteria of the genus Desulfovibrio to live in the presence of oxygen can be very useful for biological destruction NC in the effluents because, for large-scale industrial production, performing processes in the absence of air is too complicated and requires huge financial expenses. The biodegradation of NC by D. desulfuricans under aerobic conditions was studied (Khryachkov et al. 2017; Saratovskikh et al. 2018a; Saratovskikh et al. 2018b); the obtained results are presented in Table 2.
Table 2.
Contents of elements and nitrate and nitrite ions in the NC samples after incubation with D. desulfuricans (Khryachkov et al. 2017)
| Incubation time, days | рН | С, % | N, % | NO32−, μg/mL | NO22−, μg/mL | Viscosity | Density of acetone solution of NC, g/cm3 | Mn | Mw | |
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute, cP | Kinematic, cSt | |||||||||
| 0.279 (acetone) | 0.353 (acetone) | 0.791 | ||||||||
| 0 | 6.0 | n.d.* | 13.38 | 5.94–6.24 | 0 | 0.5833 | 0.7304 | 0.799 | n.d. | 324,216 |
| 5 | n.d. | 31.72 | 11.57 | 5.80 | n.d. | 1.744 | 2.179 | 0.801 | 21,100 | 114,900 |
| 16 | 8.65 | 25.97 | 11.72 | 3.23 | n.d. | 2.090 | 2.607 | 0.802 | 15,000 | 96,800 |
| 38 | 6.75 | 25.99 | 10.58 | 5.55 | 0.52 | n.d. | n.d. | n.d. | 14,760 | 96,000 |
| 65 | 5.97 | 25.80 | 11.40 | 7.58 | 1.6 | 2.432 | 3.031 | 0.803 | 9340 | 58,000 |
*n.d. means data was not determined
Mn, number average molecular weight; Mw, weight average molecular weight
The process of biological oxidation of NC can occur in two ways – by splitting off the NO2−/NO3− groups and by breaking the C-C bond. The content of nitrate/nitrite ions in the growth medium is evidence of the occurrence of the first direction of the reaction, while the change in viscosity is a demonstration of the microorganism’s ability to break the C-C bond. Polymer macromolecules in solutions exist in swelled-coiled form. Macromolecules move faster in the solvent, reducing the coefficient of internal friction and leading to a decrease in the viscosity of the solution. No nitrite ions were found in an aqueous solution of the initial sample of NC, and the content of nitrate ions was 5.94–6.24 μg/mL. This indicates the elimination of low-molecular-weight fragments or detachment of NO32– groups. When transiting to the aqueous medium, the nitrate ion forms nitric and nitrous acids in the equilibrium reaction with water. The pH values shifted from 6.0 to 8.65 and again to 5.97 during incubation. The decrease in nitrogen and carbon content in the NC was insignificant. The decrease in the nitrogen content does not exceed 3% compared to the initial NC. The viscosity increased with the elongation of the incubation time. D. desulfuricans acted in such a way that the molecular weight distribution (MWD) changed substantially and regularly with an increase in the exposure time: The weight average molecular weight (Mw) of the polymer decreased, as well as the number average molecular weight (Mn) of the polymer. The fraction of the low-molecular-weight products increased from 4 to 14%, nearly linear. A decrease in Mw value by 2 times to the 65th day of incubation indicated the decomposition of the hydrocarbon skeleton under the action of D. desulfuricans. However, the decrease was nonlinear, and the rate of NC decomposition was maximal after the first 16 days and decreases to the 65th day of incubation. A comparison of the series with different biodegradation times within 65 days clearly demonstrates that the molecular weight characteristics of the samples after the biological treatment with SRB change insignificantly with the elongation of the incubation time (Table 2).
The variations of pH, changes in the viscosity of solutions, and an increase in the fraction of low-molecular-weight products with increasing incubation time on the bacteria in the studied samples showed that the biooxidation of NC occurred under the action of the bacteria. However, a decrease in the accumulation rate of the low-molecular-weight products with incubation time and the corresponding decrease in the biooxidation rate indicated that the bacteria D. desulfuricans lose activity possibly due to the protooxidation of the reaction solution, or the complete consumption of the nutrient medium necessary for the vital activity of the bacteria, or die off of some bacteria. This fact should be taken into account when developing the technological biological decontamination of effluents from NC production.
Now then, the effects of NC biooxidation using bacteria D. desulfuricans were manifested within the first 16–38 days. Incubation of the bacteria for more than 16 days exerted no effect on both the elimination of NO2 groups and the destruction of the hydrocarbon skeleton. Bacteria did not involve the most part of the hydrocarbon skeleton, and NO2 groups localized inside the polymer globule. Since the viscosity increased and the MWD decreased, it seems probable that the С–С bonds cleaved in the depth of the polymer globule and the C–O–NO2 bonds remained uninvolved. The decrease in the nitrogen content in the NC samples did not exceed 3.0% (Khryachkov et al. 2017).
In spite of an insignificant degree of NC decomposition under the action of bacteria D. desulfuricans, biological methods of NC oxidation should be considered most promising since they are environmentally friendly. The works aimed at searching for more efficient types of microorganisms should be continued.
Destruction of nitrocellulose with fungi
Fungi, which belong to the domain Eukarya, are ubiquitous throughout terrestrial and aquatic environments. They are able to decompose a wide range of complex organic and inorganic pollutants of both native and industrial origin due to their intra and extracellular enzymatic systems; fungi are able to attack and metabolize a wide range of complex organic compounds (Goodell et al. 2020; Vaksmaa et al. 2023). For the biological treatment of effluents from the cellulose industry, a microorganism must grow rapidly, competitively predominate in the environment, and be able to perform the metabolism of lignin and its low- and high-molecular-weight derivatives. For example, white rot fungi that can destruct lignin in the medium fulfill these requirements. Screening studies were performed to determine the most productive types of fungi for biotechnological processes. The screening of ten fungi strains (Sundaram et al. 1995) revealed more than 20% degradation of NC for Bjerkandera adusta, Acremonium persicinum, Sclerotium rolfsii, and Fusarium solani after 3 days of growth (Table 3). NC was used as the single nitrogen source with starch as the co-substrate. The most efficient destruction of NC (35%) within 3 days can be achieved using combined cultures S. rolfsii АТСС 24459 synthesizing cellulose-decomposing enzymes and denitrifying fungus F. solani IFO 31093. A sharp decrease in the pH (from 6.0 to 2.0) within 7 days was observed during the process. In such acidic media, the enzymes degrading NC were inactivated and NC degradation was inhibited. Buffering of the medium to pH 6.0 was proposed. However, a sharp decrease in the pH of the nutrient medium was observed only in the presence of S. rolfsii.
Table 3.
The capability of mycelial fungi for NC degrading singly (Sundaram et al. 1995) and in co-culture (Sharma et al. 1995a) in a liquid medium containing the co-substrate (starch 0.1 %) and NC in initial concentration 150 mg
| Microorganism | Biomass, mg of dry weight | Remained NC, mg | Remote NC, % | ||||
|---|---|---|---|---|---|---|---|
| Incubation time, days | |||||||
| 0 | 3 | 7 | 3 | 7 | 3 | 7 | |
| Control | 0 | 0 | 0 | 144.6 | 143.4 | 3.6 | 4.4 |
| Acremonium persicinum ATCC 60921 | 26.0 | 58.4 | 57.4 | 118.4 | 134.8 | 21.1 | 10.1 |
| Basidiomycetes sp. NRRL 6464 | 49.8 | 40.1 | 33.9 | 140.5 | 125.9 | 6.3 | 16.1 |
| Bjerkandera adusta ESF 620 | 27.3 | 64.9 | 53.2 | 112.8 | 125.3 | 24.8 | 17.7 |
| Cyathus stercoreus NRRL 6473 | 40.6 | 57.8 | 58.5 | 142.5 | 138.2 | 5.0 | 7.9 |
| Irpen lacteus Z 212 | 23.1 | 22.5 | 14.3 | 136.4 | 113.6 | 9.1 | 24.3 |
| Phanerochaete chrysosporium ATCC 32629 | 48.7 | 43.0 | 48.2 | 132.1 | 126.8 | 11.9 | 15.5 |
| Trametes versicolor ESF 491 | 41.7 | 46.0 | 47.9 | 128.2 | 118.2 | 14.5 | 21.2 |
| Trichoderma pseudokoningii FTK 228 | 33.6 | 33.2 | 38.0 | 124.3 | 120.8 | 17.1 | 19.5 |
| Sclerotium rolfsii ATCC 24459 | 30.8 | 83.5 | 88.4 | 118.0 | 120.8 | 21.3 | 19.4 |
| Fusarium solani IF0 31093 | 26.4 | 77.5 | 55.0 | 114.6 | 114.6 | 23.6 | 23.6 |
| S. rolfsii ATCC 24459 + F. solani IFO 31093 | 6.9 | 21.1 | 13.6* | 103.7 | 97.8* | n.d. | n.d. |
n.d., data was not determined
*Incubation time: 28 days
Since combining the cultures S. rolfsii АТСС 24459 and F. solani IF0 31093 makes it possible to aerobically decompose NC in the liquid medium (Sharma et al. 1995a), it is proposed to use this pair of white rot fungi for the purification of soils contaminated with NC.
The factors responsible for the restricted NC degradation were determined: (1) composition of the medium, (2) culture conditions, (3) accumulation of inhibition products of the key enzymes involved in degradation, and (4) functional groups in an inaccessible steric surrounding.
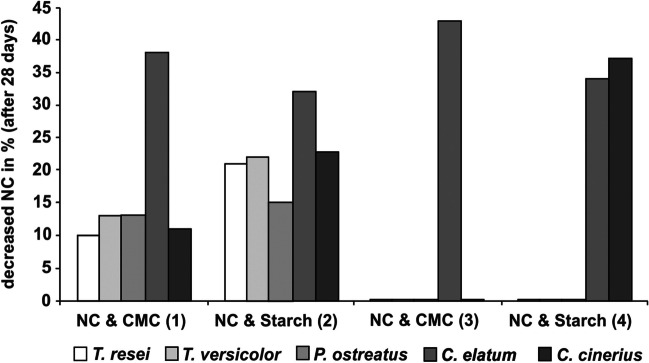
Influence on the ability to degrade nitrocellulose with different carbon and nitrogen sources was studied on five fungi: Coprinus cinereus, Chaetomium elatum, Pleurotus ostreatus, Trichoderma reesei, and Trametes versicolor (Auer et al. 2005). All studied strains of the fungi could degrade NC, but its capability of decreasing NC in the growth medium depended on the carbon and nitrogen sources. C. elatum was the only one able to use NC and carboxymethylcellulose as the single sources of nitrogen and carbon and therefore gave the highest (43%) NC decrease. C. cinereus decreased the NC content by 37% when NC was the single nitrogen source but with starch as the carbon source. NC decreases by the fungi T. versicolor, P. ostreatus, and T. reesei were 10–22% with starch as a preferred carbon source (Fig. 5).
Fig. 5.
Comparison of NC and organic nitrogen source (1, 2) and NC (3, 4) as the single nitrogen source. As carbon sources were used, carboxymethylcellulose (1, 3) and starch (2, 4) (Auer et al. 2005)
A comparison of the effects of fungi С. cinereus (NC: N = 11.9% as 3-mm granules) and C. elatum (NC: N = 12% solution in acetone) on NC showed that the “active agent” responsible for NC degradation could be enzyme(s), reactive oxygen species, or other radicals. The physical state of NC (surface area of contact with fungi) exerted a substantial effect on the degree of decreasing the content of nitrites and nitrates (Fig. 6). C. elatum was the most efficient fungus in the denitrifying and removal of NC from the suspension cultures (Auer et al. 2005).
Fig. 6.
Decrease in the NC concentration (nitrogen content lower than 12%) by fungi C. elatum and С. cinereus after 34 days of growth. Cultural media were supplemented by two different amounts of NC; cultures С, D, and Е initially contained 600 mg, and culture G contained 2000 mg (standard deviation is shown (n = 6)) (Auer et al. 2005)
The investigation of structural and thermodynamic properties of NC after treatment by C. elatum was shown to enhance the thermostability of bio-treated NC (Huang et al. 2023) and consequently its safety and expands the possibilities of NC biological treatment.
Fungus Penicillium corylophilum has previously been isolated from several sources including soil, fodders, grammas, and storage tankers for diesel fuel, but for the first time, it was isolated (Sharma et al. 1995b) from AS of the rocket fuel production effluents containing NC (nitrogen content 13.15%) and nitroglycerol. The features of the habitat polluted with NC allowed the fungus to develop effective mechanisms of utilization of NC as a single source of nitrogen. P. corylophilum could grow for 3 days and 7 days on NC (0.3%) as the single nitrogen source in the liquid medium using starch or xylan as the carbon source. Soluble nitrite was found to be completely utilized by the organism, and the glucose concentration was 10–20% of the total decrease in sugar found on the 3rd and 7th days of cultivation (Table 4).
Table 4.
Growth parameters of P. corylophilum on NC in the liquid medium (Sharma et al. 1995b)
| Microorganism | Reduced sugars, mg/mL | Glucose, mg/mL | Nitrates, mg/mL | Nitrites, mg/mL | ||||
|---|---|---|---|---|---|---|---|---|
| 3 days | 7 days | 3 days | 7 days | 3 days | 7 days | 3 days | 7 days | |
| Controla | n.d. | n.d. | 11.0 | 0.3 | 12.2 | 11.1 | 9.5 | 9.4 |
| Controlb | n.d. | n.d. | 3.4 | 1.9 | 11.4 | 11.4 | 8.6 | 8.2 |
| P. corylophiluma | 104.8 | 58.4 | 21.7 | 11.3 | 6.0 | 8.4 | 0 | 0 |
| P. corylophilumb | 39.3 | 23.2 | 4.0 | 2.8 | 9.9 | 8.7 | 0 | 0 |
aStarch was used as the co-substrate (0.1 %)
bXylan was used as the co-substrate (0.1 %)
n.d. means data was not determined. Initial pH 6.0; after 7 days of growth, the medium was brought to pH 5.5
As a rule, micromycetes possess a set of enzymes, each of which performs its specific function (Bulhak et al. 2016). Cellobiohydrolase, β-Glucosidase, and endoglucanases are key enzymes of cellulose hydrolysis (Urlacher and Koschorreck 2021). Such extracellular fungal enzymes as oxidoreductases and specifically laccases (manganese peroxidase, lignin peroxidase) are widely studied as well. However, studies (Sharma et al. 1995b) confirmed that activation of cellulolytic enzymes was not fast. For example, the cellulose-degrading enzyme β-1,4-endoglucanase was found only on the third day in the medium containing starch as the co-substrate, but the level of this enzyme decreased on the seventh day. No β-1,4-endoglucanase was found in the medium containing xylan as the co-substrate. Starch was necessary for the production of β-1,4-endoglucanase in P. corylophilum. Another enzyme decomposing cellulose, β-glucosidase, was found in the growing culture in the intra- and extracellular fractions. A significant content of intracellular enzymes of nitrate and nitrite reductases was determined in the growing culture. The contents of both enzymes increased on the 3rd day and decreased on the 7th day. It was found that 20% of NC was microbiologically utilized within 3 days. NC was assimilated more efficiently in the presence of starch. The data are given in Table 5. The combination of P. corylophilum with F. solani IF0 31093 gave no results (Sharma et al. 1995b).
Table 5.
Degradation of NC by P. corylophilum and in combination with F. solani in the liquid medium containing a co-substrate (Sharma et al. 1995b)
| Microorganism | Residual NC, mga | Dry weight of biomass, mgb | |||
|---|---|---|---|---|---|
| 0 day | 3 days | 0 day | 3 days | 7 days | |
| P. corylophilumc | 150 | 120.7 | 28.2 | 28.6 | 46.9 |
| P. corylophilum + F. solanic | 150 | 125.9 | 18.7 | 27.3 | 31.9 |
| P. corylophilud | 150 | 124.5 | 28.2 | 37.4 | 49.6 |
| P. corylophilum + F. solanid | 150 | 132.2 | 18.7 | 29.8 | 33.5 |
aThe data were corrected by the biomass coprecipitated with NC
bThe data corrected to the amount of the biomass isolated from acetone. The abiotic control on the 3rd and 7th days under other equivalent conditions resulted in the loss of NC by 1.4%
cStarch was used as the co-substrate in a concentration of 0.1%
dXylan was used as the co-substrate in a concentration of 0.1%
The general routes of biodegradation of nitro esters of explosives can be presented in the generalized form as follows (Walker and Kaplan 1992): >C–O–NO2 →~ >C–OH ~ mineralization. Nitroaromatic compounds are usually considered to be resistant to oxidation with oxygenase enzymes because of the presence of electrons easily donated by the nitro groups of the ring. Nevertheless, the oxidation of the aromatic ring (in particular, of TNT) became possible by the fungus system Phanerochaete chrysosporium. The oxidation of both nitro groups to the corresponding amino groups occurred by the reduction–oxidation system including peroxidase enzymes of P. chrysosporium.
Fungus Aspergillus fumigatus was able to utilize cellulose nitrate as a sole source of nitrogen with supplied glucose as a carbon source (Brodman and Devine 1981). An analysis of publications shows that there are a number of fungi capable of decomposing NCs. Summarized data are presented in Table 6.
Table 6.
Degree of degradation of NC by various fungi at NC content 11.0-13.4%
| Microorganism | Incubation time, days | Degree of NC degradation, % | Reference |
|---|---|---|---|
| Acremonium persicinum ATCC 60921 | 7 | 10.1 | Sundaram et al. (1995) |
| Basidiomycetes sp. NRRL 6464 | 7 | 16.1 | Sundaram et al. (1995) |
| Bjerkandera adusta ESF 620 | 7 | 17.7 | Sundaram et al. (1995) |
| Coprinus cinereus | 28 | 37 | Auer et al. (2005) |
| Chaetomium elatum | 28 | 43.0 | |
| Chaetomium elatum | 6 | 10.33 | Huang et al. (2023) |
| Cyathus stercoreus NRRL 6473 | 7 | 7.9 | Sundaram et al. (1995) |
| Irpex lacteus Z 212 | 7 | 24.3 | Sundaram et al. (1995) |
| Fusarium solani IF0 31093 | 7 | 23.6 | Sundaram et al. (1995) |
| Penicillium corylophilum | 3–7 | 20.0 | Sharma et al. (1995b) |
| Phanerochaete chrysosporium ATCC 32629 | 7 | 15.5 | Sundaram et al. (1995) |
| Pleurotus ostreatus | 12.0–15.0 | Auer et al. (2005) | |
| Sclerotium rolfsii ATCC 24459 | 7 | 19.4–31.0 | Sundaram et al. (1995) |
| S. rolfsii ATCC 24459+ F. solani | 3 | 38.0 | Sharma et al. (1995a) |
| Trametes versicolor ESF 491 | 7 | 12.0–21.2 | Sundaram et al. (1995) |
| Trichoderma pseudokoningii FTK 228 | 7 | 19.5 | Sundaram et al. (1995) |
| Trichoderma reesei | 7 | 10.0-22.0 | Auer et al. (2005) |
An effluent of industrial NC production is a mixture of many components, including compounds containing metals. It is found for the enzymes produced by Aspergillus niger (Bulhak et al. 2016) that the coordination compounds of Fe(III) with hydrazines and sulfodiazines as ligands inhibit the biosynthesis of hydrolases. The complexes substantially suppress cellobiohydrolase, endoglucanase, xylanase, and β-glucosidase (to a lesser extent). The compound containing chlorine ions exerts a stronger inhibition effect, and the compound-bearing NO3 ions showed the weakest inhibition effect.
Recently, the biological oxidation of NC under aerobic conditions was studied with mycelial fungus Fusarium solani (Saratovskikh et al. 2018a; Saratovskikh et al. 2018b). The results of the measurements performed during NC incubation on F. solani are presented in Table 7. It is seen that the pH value changes insignificantly and nonuniformly to the acidic values with an increase in the incubation time. This was first related to a complicated globular tangled secondary and tertiary structure of NC and showed that it is difficult for microorganisms to get into deep layers of NC; i.e., their action was restricted, most likely, by surface layers. A change in the nitrogen content was also nonuniform. The lowest values were established on the 16th and 38th days of incubation. The decrease in the nitrogen content was 2.77% and 2.87%, respectively.
Table 7.
Contents of elements, nitrate, and nitrite ions in the NC samples after incubation with Fusarium solani (Saratovskikh et al. 2018b)
| Exposure time, days | рН | С, % | N, % | NO32−, μg/mL | NO22−, μg/mL | Viscosity | Mn | Mw | |
|---|---|---|---|---|---|---|---|---|---|
| Absolute, cP | Kinematic, cSt | ||||||||
| 0.279 (acetone) | 0.353 (acetone) | n.d. | n.d. | ||||||
| 0 | 7.0 | n.d.* | 13.38 | n.d. | n.d. | n.d | n.d | n.d. | 324216 |
| 5 | 7.80 | 30.19 | 11.76 | 11.40 | 3.34 | 2.413 | 3.011 | 15100 | 48800 |
| 16 | 8.40 | 25.14 | 10.61 | 5.15 | 0.52 | 2.314 | 2.884 | 16100 | 37300 |
| 38 | 5.56 | 26.34 | 10.51 | 2.78 | 0.22 | n.d. | n.d. | n.d. | n.d. |
| 65 | 6.53 | 25.90 | 11.78 | 4.04 | 0.42 | 2.527 | 3.148 | 20000 | 60900 |
n.d., data was not determined; Mn, number average molecular weight; Mw, weight average molecular weight
Evidently, nitrate and nitrite groups were formed with nitrogen release. The values measured in the solution are presented in Table 7. The measured amount of nitrite groups was maximal within the first 5 days of incubation (3.34 μg/mL), whereas this amount for longer terms was insignificant. The amount of nitrate groups measured in the solution changes similarly: The maximum value within the first 5 d of incubation was 11.40 μg/mL, and furthermore, the concentration of nitrate groups decreased substantially. Naturally, after 16 days, changes in the concentration of nitrite groups were parallel with changes in the pH of the solution.
The biological oxidation of NC could occur via two pathways: elimination of NO2– groups and cleavage of the C–C bond. If the content of nitrate and nitrite ions could serve as proof that the reaction proceeds via the first route, then a change in the viscosity was obviously related to a change in the molecular weight of NC and could demonstrate the ability of microorganisms to cleave this bond. Under the action of F. solani, the viscosity of the solutions insignificantly increased with an increase in the incubation time (Table 7).
One of the most important characteristics of a polymer is molecular weight distribution (MWD). As can be seen from Table 7, the MWD values demonstrated the presence of insignificant amounts of low-molecular-weight fractions, the percentage content of which changes slightly with incubation time on F. solani.
The heat release was principally significant for the estimation of the degree of decomposition of NC. Figure 7 showed the obtained dependences of the heat release rate on the incubation time (Saratovskikh et al. 2018a). The decomposition of both initial NC and treated with F. solani has been studied. The total heat release of the initial NC was an average of 975 cal/g, while it is 770 cal/g for the NC after incubation with F. solani for 36 days. A longer incubation did not lead to an increase in the heat release rate (Saratovskikh et al. 2018a). A comparison of this result with the data of another work (Saratovskikh et al. 2018b) showed that the incubation of NC with fungi F. solani resulted in a lower heat of thermal decomposition and, hence, in a lower nitrogen content in the NC. However, as a result of a long-term or severe storage process environment, a slow thermal decomposition of NC can take place (Li et al. 2022).
Fig. 7.

Time dependences of the heat release rate upon the thermal decomposition of NC samples at 139.8 °С: (1) initial NC, (2) NC incubated with F. solani for 36 days, and (3) NC incubated with F. solani for 65 days
The results of the studies carried out convincingly showed that the incubation of NC with mycelial fungus F. solani makes it possible to reach a high degree of oxidation. The nitrogen content decreased by 3%, the molecular weight decreased, the polymer structure changed substantially, and the heat release decreased significantly during incubation. All these facts indicated a significant degree of biodegradation of NC during incubation on F. solani. It should be mentioned that the observed changes were much more significant than those for the incubation of NC on D. desulfuricans (Khryachkov et al. 2017) within the corresponding time intervals. However, the maximum effect was achieved within the first 16 days of incubation, and the further increase in time does not result in an increase in the effect.
Treatment of nitrocellulose industry wastewaters
Recently, the problem of industrial wastewater treatment from NC has become extremely relevant. A complete treatment system, including acid hydrolysis, electrodialysis, and anaerobic processing using biological destruction, proved to be a technically feasible alternative to convert waste nitrocellulose into innoxious products (Tai 1996).
Alkaline hydrolysis was considered to be preferable from the viewpoint of the safety of workers (Kolmakov et al. 2017). Several reactions occur simultaneously during the alkaline destruction of NC:
denitrification of nitro esters with the formation of the starting products;
hydrolysis of glycoside bonds with the decomposition of macromolecules; and
cleavage of pyranose cycles and oxidation of glycoside residues.
The scheme of saponification can be presented as follows:
The regression equations relate the reaction conditions to the degree of destruction of NC.
The oxidative degradation of toxic NC acid wastewater by using low-grade MnO2 ore was investigated (Zhang et al. 2017). Bioremediation technology by composting soils contaminated with NC and TNT also showed positive results (Williams et al. 1992). A hybrid approach for the destruction of NC-containing sewage sludge from a real chemical industrial complex including chemical alkaline hydrolysis and mesophilic anaerobic digestion allowed to achieve the balance between environmental safety and economic efficiency (Gaydamaka et al. 2023). In addition, other methods of chemical and biological treatment of wastewater containing nitrocellulose continue to be developed (El-Diwani et al. 2009; Pouretedal et al. 2021; Ugurlu and Ozturkcu 2018).
The obtained non-toxic, biodegradable products of the chemical NC decomposition can be used as mineral and organic fertilizers, deoxidizers of acidic soils, and absorbents of acidic industrial gasses and can be discharged onto the field together with silt residues of water effluents or to dung receivers (Zhang et al. 2022).
However, the presently known chemical methods for NC degradation and processing of industrial effluents, such as alkaline or acidic hydrolysis or combustion, are evaluated by specialists as expensive and unsustainable (Wang et al. 1982). Therefore, it is necessary to continue efforts to search for microorganisms from various habitats for a more successful impact on NC in order to create an effective technology for the biodegradation of these wastes.
Conclusions
The first review of microbiological methods for the decomposition of nitrocellulose, including methods for treating effluents from the production of NC in wastewater, was presented. Based on the foregoing, the biodegradation of NS has great perspectives. Further research of new metabolic pathways will improve our understanding of NC biotransformation. The development of biocatalytic processes that use cheap waste feedstock to produce commercially useful products has the potential to reduce waste and efforts in biotransformation. A combination of increasing commercial interest and advances in understanding the genetic and biochemical basis of biodegradation is expected to produce a more rational approach to wastewater treatment technology.
Abbreviations
- NC
nitrocellulose
- TNT
trinitrotoluene
- SRB
sulfate-reducing bacteria
Author contributions
All authors contributed to the study’s conception and design. All authors read and approved the final manuscript.
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation: State Assignment No. АААА-А19-119071890015-6 and project No. 075-15-2021-1051.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anasonye F, Winquist E, Räsänen M, Kontro J, Björklöf K, Vasilyeva G, Jørgensen KS, Steffen KT, Tuomela M. Bioremediation of TNT contaminated soil with fungi under laboratory and pilot scale conditions. Int Biodeterior Biodegradation. 2015;105:7–12. doi: 10.1016/j.ibiod.2015.08.003. [DOI] [Google Scholar]
- Auer N, Hedger JN, Evans CS. Degradation of nitrocellulose by fungi. Biodegradation. 2005;16:229–236. doi: 10.1007/s10532-004-0896-9. [DOI] [PubMed] [Google Scholar]
- Berthumeyrie S, Collin S, Bussiere P-O, Therias S. Photooxidation of cellulose nitrate: new insights into degradation mechanisms. J Hazard Mater. 2014;272:137–147. doi: 10.1016/j.jhazmat.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Brodman BW, Devine MP. Microbial attack of nitrocellulose. J Appl Polym Sci. 1981;26(3):997–1000. doi: 10.1002/app.1981.070260322. [DOI] [Google Scholar]
- Bulhak I, Ciloci DA, Bourosh P, Tiurina J, Bologa O, Bivol C, Clapco S, Verejan A, Labliuc S, Danilescu O. Structure and some biological properties of Fe(III) complexes with nitrogen-containing ligands. Chem J Moldova. 2016;11(1):39–49. doi: 10.19261/cjm.2016.11(1).05. [DOI] [Google Scholar]
- Cadby IT, Faulkner M, Cheneby J, Long J, Van Helden J, Dolla A, Cole JA. Coordinated response of the Desulfovibrio desulfuricans 27774 transcriptome to nitrate, nitrite and nitric oxide. Sci Rep. 2017;7(1):16228. doi: 10.1038/s41598-017-16403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C, Romao MJ. Structural and mechanistic insights on nitrate reductases. Protein Sci. 2015;24(12):1901–1911. doi: 10.1002/pro.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Bak F. Nitrate reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil: sulfide inhibition, kinetics, and regulation. Appl Environ Microbiol. 1994;60(1):291–297. doi: 10.1128/aem.60.1.291-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Diwani G, El-Ibiari N, Hawash S. Treatment of hazardous wastewater contaminated by nitrocellulose. J Hazard Mater. 2009;167(1-3):830–834. doi: 10.1016/j.jhazmat.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Fournier M, Dermoun Z, Durand M-C, Dolla A. A new function of the Desulfovibrio vulgaris Hildenborough [Fe] hydrogenase in the protection against oxidative stress. J Biol Chem. 2004;279(3):1787–1793. doi: 10.1074/jbc.M307965200. [DOI] [PubMed] [Google Scholar]
- Freedman DL, Caenepeel BM, Kim BJ. Biotransformation of nitrocellulose under methanogenic conditions. Water Sci Technol. 1996;34(5-6):327–334. doi: 10.1016/0273-1223(96)00662-2. [DOI] [Google Scholar]
- Freedman DL, Cashwell JM, Kim BJ. Biotransformation of explosive-grade nitrocellulose under denitrifying and sulfidogenic conditions. Waste Manag. 2002;22(3):283–292. doi: 10.1016/S0956-053X(01)00032-0. [DOI] [PubMed] [Google Scholar]
- French CE, Nicklin S, Bruce NC. Aerobic degradation of 2, 4, 6-trinitrotoluene by Enterobacter cloacae PB2 and by pentaerythritol tetranitrate reductase. Appl Environ Microbiol. 1998;64(8):2864–2868. doi: 10.1128/AEM.64.8.2864-2868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydamaka S, Gladchenko M, Maslova O, Senko O, Kornilova A. Kornilov I (2023) Application of the hybrid chemical-biocatalytic approach for conversion of nitrocellulose-containing sewage sludge. Processes. 2023;11(7):2017. doi: 10.3390/pr11072017. [DOI] [Google Scholar]
- Giacomucci L, Toja F, Sanmartín P, Toniolo L, Prieto B, Villa F, Cappitelli F. Degradation of nitrocellulose-based paint by Desulfovibrio desulfuricans ATCC 13541. Biodegradation. 2012;23:705–716. doi: 10.1007/s10532-012-9546-9. [DOI] [PubMed] [Google Scholar]
- Goodell B, Winandy JE, Morrell JJ. Fungal degradation of wood: emerging data, new insights and changing perceptions. Coatings. 2020;10(12):1210. doi: 10.3390/coatings10121210. [DOI] [Google Scholar]
- Gupta S, Goel SS, Siebner H, Ronen Z, Ramanathan G. Transformation of 2,4,6-trinitrotoluene by Stenotrophomonas strain SG1 under aerobic and anaerobic conditions. Chemosphere. 2023;311:137085. doi: 10.1016/j.chemosphere.2022.137085. [DOI] [PubMed] [Google Scholar]
- Habineza A, Zhai J, Mai T, Mmereki D, Ntakirutimana T. Biodegradation of 2, 4, 6- trinitrotoluene (TNT) in contaminated soil and microbial remediation options for treatment. Periodica Polytechnica Chem Eng. 2016;61(3):171–187. doi: 10.3311/PPch.9251. [DOI] [Google Scholar]
- Haïdour A, Ramos JL. Identification of products resulting from the biological reduction of 2, 4, 6-trinitrotoluene, 2, 4-dinitrotoluene, and 2, 6-dinitrotoluene by Pseudomonas sp. Environ Sci Technol. 1996;30(7):2365–2370. doi: 10.1021/es950824u. [DOI] [Google Scholar]
- Huang J, Zhang A, Xue H, Zhou J, Ding Y, Xiao Z (2023) Biological treatment of nitrocellulose: investigation on structural and thermodynamic properties. Preprint from Res Sq. 10.21203/rs.3.rs-3039202/v1
- Jaafaryneya M, Amani J, Halabian R. Biodegradation of 2,4,6-trinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine by Actinomycetes species, first time isolated and characterized from water, wastewater, and sludge. Water Environ J. 2023;37(3):538–548. doi: 10.1111/wej.12857. [DOI] [Google Scholar]
- Jain M, Zinjarde S, Deobagkar D, Deobagkar D. 2, 4, 6-Trinitrotoluene transformation by a tropical marine yeast, Yarrowia lipolytica NCIM 3589. Mar Pollut Bull. 2004;49(9-10):783–788. doi: 10.1016/j.marpolbul.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Khryachkov V, Saratovskikh E, Yarullin R, Kulikov A. Effect of the D. desulfuricans bacterium and UV radiation on nitrocellulose oxidation. Russian J Phys ChemB. 2017;11:697–703. doi: 10.1134/S1990793117040169. [DOI] [Google Scholar]
- Kim H-Y, Bennett GN, Song H-G. Degradation of 2, 4, 6-trinitrotoluene by Klebsiella sp. isolated from activated sludge. Biotechnol Lett. 2002;24:2023–2028. doi: 10.1023/A:1021127201608. [DOI] [Google Scholar]
- Kim H-Y, Song H-G. Transformation and mineralization of 2, 4, 6-trinitrotoluene by the white rot fungus Irpex lacteus. Appl Microbiol Biotechnol. 2003;61:150–156. doi: 10.1007/s00253-002-1211-5. [DOI] [PubMed] [Google Scholar]
- Kolmakov K, Kozlov G, Rozen A, Roshchin A, Bloshenko A. Chemical recycling of cellulose nitrate waste. Russian J Phys Chem B. 2017;11:691–696. doi: 10.1134/S1990793117040182. [DOI] [Google Scholar]
- Kurinenko BM, Denivarova NA, Davydov RE, Yakovleva GY. Features of the toxic action of 2,4,6-trinitrotoluene on Escherichia coli K12. Appl Biochem Microbiol. 2007;43(1):52–56. doi: 10.1134/s0003683807010097. [DOI] [PubMed] [Google Scholar]
- Li S, Li Y, Ding Y, Yuan Y, Xiao Z. Chemical modification of nitrocellulose by grafting sodium carboxymethyl. Cellulose. 2022;29:8103–8115. doi: 10.1007/s10570-022-04780-7. [DOI] [Google Scholar]
- Liu J. Nitrate Esters Chemistry and Technology. Singapore: Springer; 2019. Nitrocellulose; pp. 469–580. [Google Scholar]
- Lobo SA, Melo AM, Carita JN, Teixeira M, Saraiva LM. The anaerobe Desulfovibrio desulfuricans ATCC 27774 grows at nearly atmospheric oxygen levels. FEBS Lett. 2007;581(3):433–436. doi: 10.1016/j.febslet.2006.12.053. [DOI] [PubMed] [Google Scholar]
- Marietou A, Richardson D, Cole J, Mohan S. Nitrate reduction by Desulfovibrio desulfuricans: a periplasmic nitrate reductase system that lacks NapB, but includes a unique tetraheme c-type cytochrome. NapM FEMS Microbiol Lett. 2005;248(2):217–225. doi: 10.1016/j.femsle.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Pak JW, Knoke KL, Noguera DR, Fox BG, Chambliss GH. Transformation of 2, 4, 6-trinitrotoluene by purified xenobiotic reductase B from Pseudomonas fluorescens I-C. Appl Environ Microbiol. 2000;66(11):4742–4750. doi: 10.1128/AEM.66.11.4742-4750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova OE, Tarasova NB, Davydova MN. Biotechnological potential of sulfate-reducing bacteria for transformation of nitrocellulose. Anaerobe. 2002;8(6):315–317. doi: 10.1016/s1075-9964(03)00029-5. [DOI] [PubMed] [Google Scholar]
- Pouretedal HR, Shamsi M, Arabiyan D. Statistical optimization of nitrocellulose removal from industrial wastewater by electrocoagulation using response surface method. Desalin Water Treat. 2021;212:212–219. doi: 10.5004/dwt.2021.26630. [DOI] [Google Scholar]
- Ribeiro EN, Da Silva FT, De Paiva TCB. Ecotoxicological evaluation of waste water from nitrocellulose production. J Environ Sci Health, Part A: Toxic/Hazardous Subst Environ Eng. 2013;48(2):197–204. doi: 10.1080/10934529.2012.717812. [DOI] [PubMed] [Google Scholar]
- Saratovskikh E, Avdeeva L, Yarullin R, Kazakov A. Evaluation of denitration of nitrocellulose by microbiological treatment for industrial waste effluents using calorimetry analysis. J Therm Anal Calorim. 2018;134:653–664. doi: 10.1007/s10973-018-7658-0. [DOI] [Google Scholar]
- Saratovskikh E, Shcherbakova V, Yarullin R. Nitrocellulose degradation by the fungus Fusarium solani. Appl Biochem Microbiol. 2018;54(1):45–52. doi: 10.1134/S0003683818010106. [DOI] [Google Scholar]
- Serrano-González MY, Chandra R, Castillo-Zacarias C, Robledo-Padilla F, de Rostro-Alanis MJ, Parra-Saldivar R. Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction. Defence Technol. 2018;14:151–164. doi: 10.1016/j.dt.2018.01.004. [DOI] [Google Scholar]
- Sharma A, Sundaram S, Zhang YZ, Brodman B. Biodegradation of nitrate esters. II. Degradation of nitrocellulose by a fungus isolated from a double-base propellant. J Appl Polym Sci. 1995;55(13):1847–1854. doi: 10.1002/app.1995.070551315. [DOI] [Google Scholar]
- Sharma A, Sundaram ST, Zhang Y-Z, Brodman BW. Nitrocellulose degradation by a coculture of Sclerotium rolfsii and Fusarium solani. J Ind Microbiol. 1995;15(1):1–4. doi: 10.1007/BF01570005. [DOI] [Google Scholar]
- Spanggord RJ, Spain J, Nishino S, Mortelmans K. Biodegradation of 2, 4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol. 1991;57(11):3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan F, Simon L, Ioannidis N, Patel S, Ophir Z, Gogos C, Jaffe M, Tirmizi S, Bonnett P, Abbate P (2020) Chemical reaction modeling of industrial scale nitrocellulose production for military applications. AICHE J 66(7). 10.1002/aic.16234
- Sundaram S, Zhang Y, Sharma A, Ng K, Brodman B. Screening of mycelial fungi for nitrocellulose degradation. J Appl Polym Sci. 1995;58(12):2287–2291. doi: 10.1002/app.1995.070581216. [DOI] [Google Scholar]
- Tai F-J. Anaerobic digestion and acid hydrolysis of nitrocellulose. Dissertations New Jersey Institute of Technology; 1996. [Google Scholar]
- Ugurlu A, Ozturkcu SD. Treatment of nitrocellulose industry watewaters by upflow denitrification filter: effect of packing media and recirculation. Environ Proc. 2018;5:81–94. doi: 10.1007/s40710-017-0282-3. [DOI] [Google Scholar]
- Urlacher VB, Koschorreck K. Pecularities and applications of aryl-alcohol oxidases from fungi. Appl Microbiol Biotechnol. 2021;105:4111–4126. doi: 10.1007/s00253-021-11337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaksmaa A, Guerrero-Cruz S, Ghosh P, Zeghal E, Hernando-Morales V, Niemann H (2023) Role of fungi in bioremediation of emerging pollutants. Front Mar Sci. 10.3389/fmars.2023.1070905
- Vorbeck C, Lenke H, Fischer P, Spain JC, Knackmuss H-J. Initial reductive reactions in aerobic microbial metabolism of 2, 4, 6-trinitrotoluene. Appl Environ Microbiol. 1998;64(1):246–252. doi: 10.1128/AEM.64.1.246-252.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Kaplan DL. Biological degradation of explosives and chemical agents. Biodegradation. 1992;3(2-3):369–385. doi: 10.1016/0958-1669(92)90100-W. [DOI] [Google Scholar]
- Wang LK, Pressman M, Shuster WW, Shade RW, Bilgen F, Lynch T. Separation of nitrocellulose fine particles from industrial effluent with organic polymers. Can J Chem Eng. 1982;60(1):116–122. doi: 10.1002/cjce.5450600120. [DOI] [Google Scholar]
- White GF, Snape JR. Microbial cleavage of nitrate esters: defusing the environment. J Gen Microbiol. 1993;139(9):1947–1957. doi: 10.1099/00221287-139-9-1947. [DOI] [PubMed] [Google Scholar]
- Williams RT, Ziegenfuss PS, Sisk WE. Composting of explosives and propellant contaminated soils under thermophilic and mesophilic conditions. J Ind Microbiol. 1992;9(2):137–144. doi: 10.1007/bf01569746. [DOI] [Google Scholar]
- Zaripov SA, Naumov AV, Suvorova ES, Garusov AV, Naumova RP. Initial stages of 2,4,6-trinitrotoluene transformation by microorganisms. Microbiology. 2004;73(4):398–403. doi: 10.1023/b:mici.0000036983.04480.19. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cai J, Dan Z, Tian Y, Duan N, Xin B. Simultaneous oxidative degradation of toxic acid wastewater from production of nitrocellulose and release of Mn2+ from low-grade MnO2 ore as oxidant. J Chem Technol Biotechnol. 2017;92(7):1638–1644. doi: 10.1002/jctb.5159. [DOI] [Google Scholar]
- Zhang Z, Malik MZ, Khan A, Ali N, Malik S, Bilal M. Environmental impacts of hazardous waste, and management strategies to reconcile circular economy and eco-sustainability. Sci Total Environ. 2022;807:150856. doi: 10.1016/j.scitotenv.2021.150856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Not applicable.