Abstract
Assimilatory power was measured in ten C3 species by means of a rapid-response gas exchange device as the total amount of CO2 fixed in N2-CO2 atmosphere after switching the light off. Different steady-state levels of the assimilatory power were obtained by varying light intensity and O2 and CO2 concentrations during the preexposition periods in the leaf chamber.
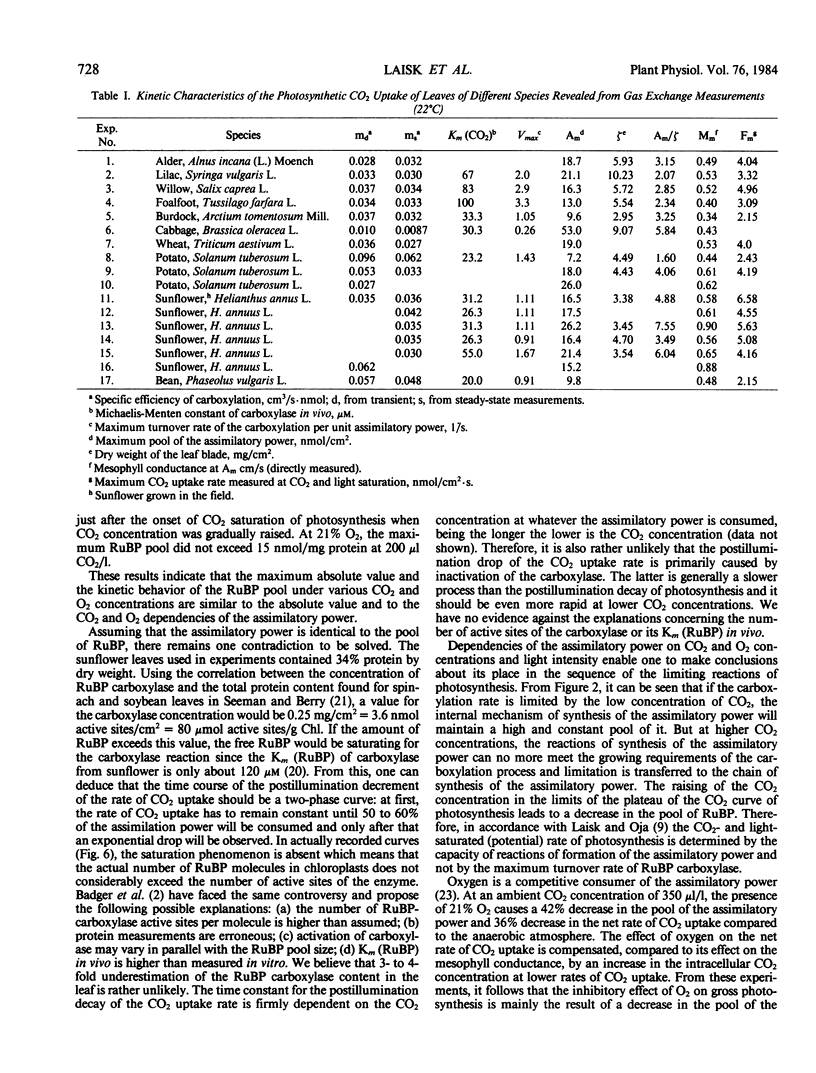
Within the limits of the linear part of the CO2 curve of photosynthesis in N2, the assimilatory power is constant, being sufficient for the assimilation of about 20 nanomoles CO2 per square centimeter leaf. The pool starts to decrease with the onset of the CO2 saturation of photosynthesis. Increase in O2 concentration from 0 to 100% at 350 microliters CO2 per liter produces a considerable decrease in the assimilatory power.
The mesophyll conductance (M) was found to be proportional to the assimilatory power (A): M = mA. The most frequently occurring values of the proportionality constant (m) (called the specific efficiency of carboxylation) were concentrated between 0.03 and 0.04 centimeter per second per nanomole A per square centimeter but the measured extreme values were 0.01 and 0.06 centimeter per second per nanomole A per square centimeter. The specific rate of carboxylation (the rate per unit A) showed a hyperbolic dependence on CO2 conentration with the most frequent values of Km (CO2) ranging from 25 to 35 micromolar in the liquid phase of mesophyll cells (extremes 23 and 100 micromolar).
It is concluded that the CO2− and light-saturated rate of photosynthesis is limited by the reactions of the formation of the assimilatory power and not by ribulose-1,5-bisphosphate carboxylase. O2 is a competitive consumer of the assimilatory power, and the inhibitory effect of O2 on photosynthesis is caused mainly by a decrease in the pool of the assimilatory power at high O2 concentrations. In intact leaves, the kinetic properties of ribulose-1,5-bisphosphate carboxylase seem to be variable.
Full text
PDF
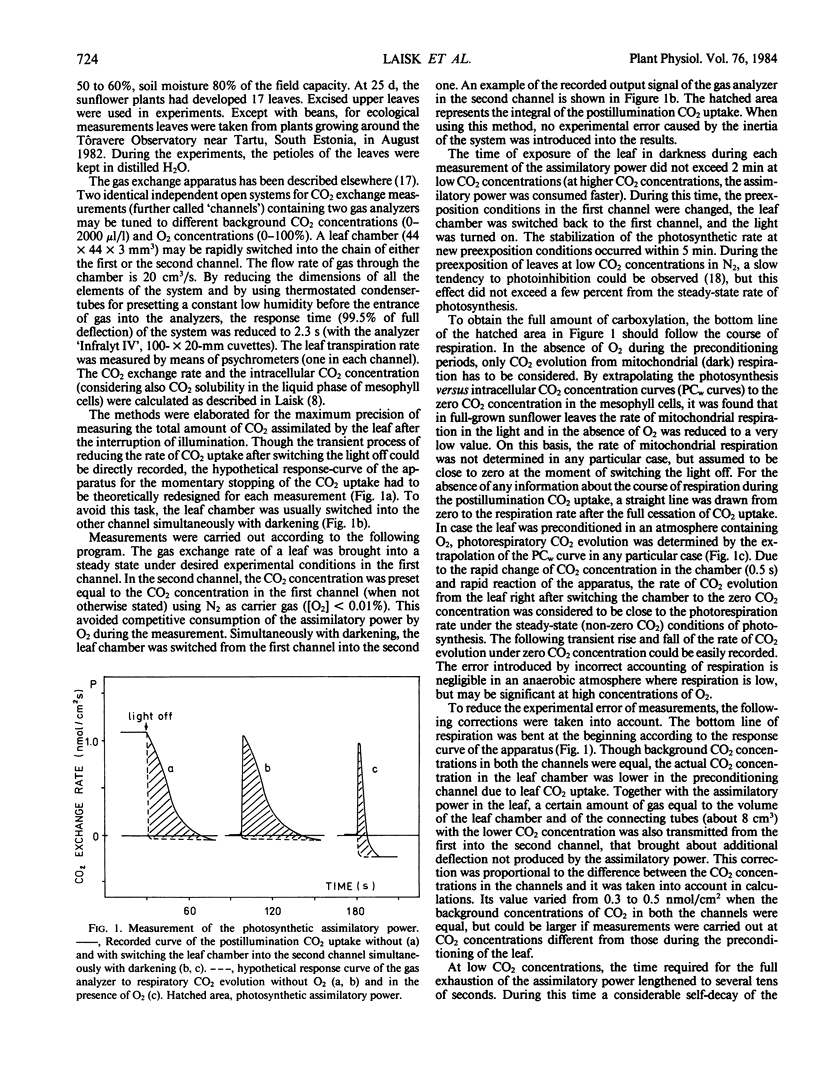
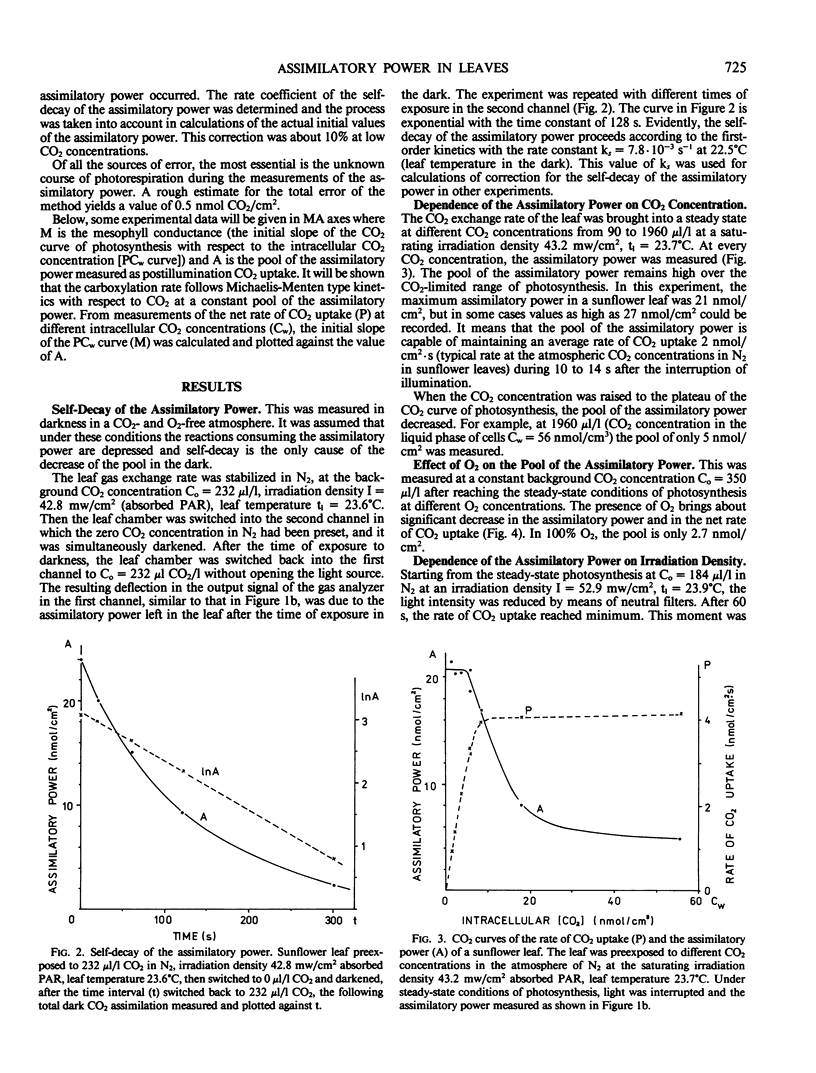
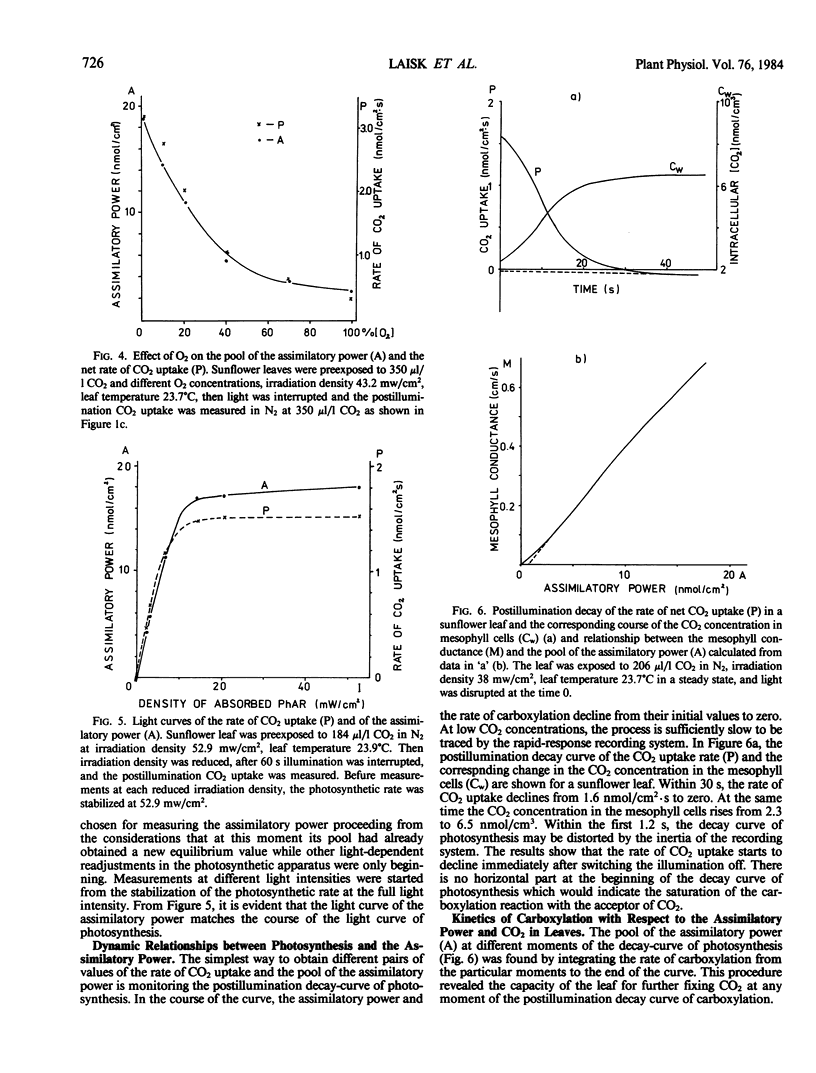
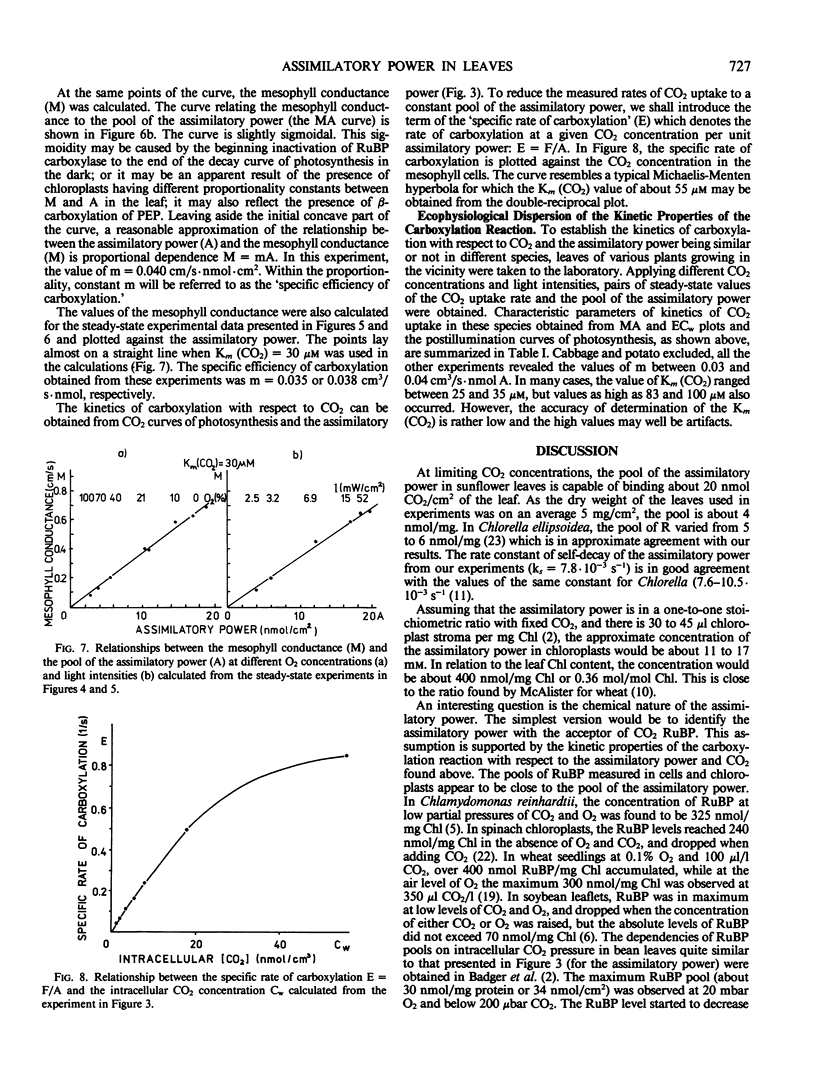

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., Whatley F. R., Allen M. B. Assimilatory Power in Photosynthesis: Photosynthetic phosphorylation by isolated chloroplasts is coupled with TPN reduction. Science. 1958 May 2;127(3305):1026–1034. doi: 10.1126/science.127.3305.1026. [DOI] [PubMed] [Google Scholar]
- Calvin M., Benson A. A. The Path of Carbon in Photosynthesis. Science. 1948 May 7;107(2784):476–480. doi: 10.1126/science.107.2784.476. [DOI] [PubMed] [Google Scholar]
- Hitz W. D., Stewart C. R. Oxygen and Carbon Dioxide Effects on the Pool Size of Some Photosynthetic and Photorespiratory Intermediates in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1980 Mar;65(3):442–446. doi: 10.1104/pp.65.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogetsu D., Miyachi S. Effect of Oxygen on the Light-enhanced Dark Carbon Dioxide Fixation in Chlorella Cells. Plant Physiol. 1970 Feb;45(2):178–182. doi: 10.1104/pp.45.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIHEI T., SASA T., MIYACHI S., SUZUKI K., TAMIYA H. Change of photosynthetic activity of Chorella cells during the course of their normal life cycle. Arch Mikrobiol. 1954;21(2):156–166. [PubMed] [Google Scholar]
- OH-HAMA T., MIYACHI S. Effects of illumination and oxygen supply upon the levels of pyridine nucleotides in Chlorella cells. Biochim Biophys Acta. 1959 Jul;34:202–210. doi: 10.1016/0006-3002(59)90248-3. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Hatch A. L., Stumpf D. K., Jensen R. G. Ribulose 1,5-bisphosphate and activation of the carboxylase in the chloroplast. Plant Physiol. 1981 Jul;68(1):252–255. doi: 10.1104/pp.68.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh H. H., Badger M. R., Watson L. Variations in K(m)(CO(2)) of Ribulose-1,5-bisphosphate Carboxylase among Grasses. Plant Physiol. 1980 Dec;66(6):1110–1112. doi: 10.1104/pp.66.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh H. H., Badger M. R., Watson L. Variations in Kinetic Properties of Ribulose-1,5-bisphosphate Carboxylases among Plants. Plant Physiol. 1981 Jun;67(6):1151–1155. doi: 10.1104/pp.67.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]


