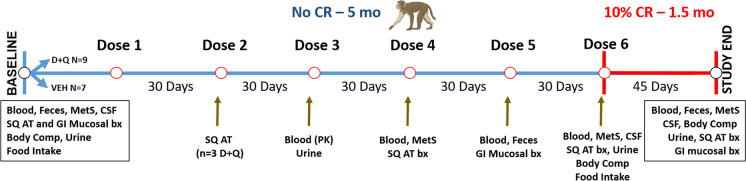
Fig. 1.
Overview of study timeline and outcome measures. Demographic details are shown in Supplemental Table 1. Seven vehicle (VEH) treated control and nine Dasatinib (D) and Quercetin (Q) treated middle-aged cynomolgus macaques were included in study. Study outcomes and timing are shown relative to ad libitum (No CR) and caloric restriction (CR) phases. Samples were collected and included those relating to metabolic syndrome (MetS) criteria, cerebrospinal fluid (CSF), subcutaneous (SQ) adipose, and gastrointestinal (GI) colon mucosal biopsies (bx), feces and blood. Computed tomography imaging was performed to determine body composition (Body Comp) to confirm the effects of the feeding phases