Abstract
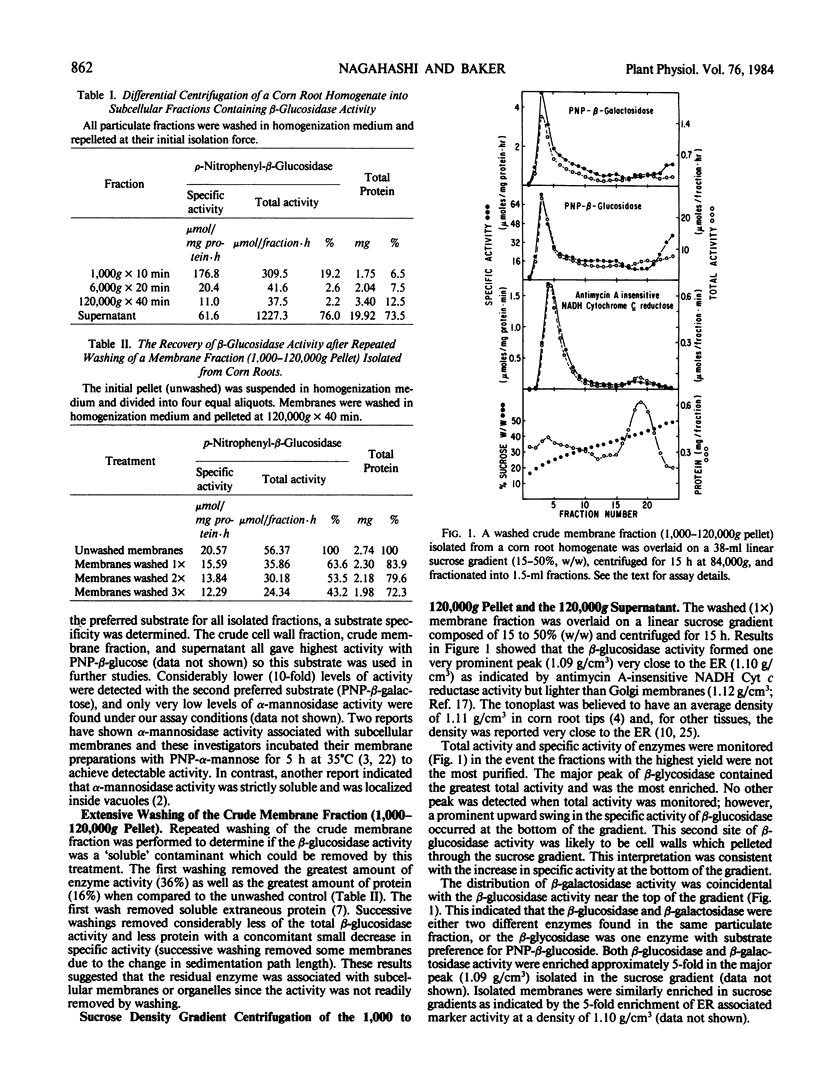
Preliminary results from differential centrifugation experiments, washing treatments, and enrichment in linear sucrose gradients at a density of 1.09 grams per cubic centimeter all indicated that β-glucosidase activity in corn root homogenates was associated with a membrane such as tonoplast. A subsequent sucrose density gradient centrifugation time course showed that the β-glucosidase was actually a soluble enzyme which moved into the gradients. The problem of soluble enzymes contaminating light density membranes in sucrose gradients and the question of centrifugation time necessary for membrane vesicles to reach isopycnic conditions are addressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford A. E. Histochemical localization of beta-glycosidases in roots of Zea mays. I. A simultaneous coupling azo-dye technique for the localization of beta-glucosidase and beta-galactosidase. Protoplasma. 1970;71(3):281–293. doi: 10.1007/BF01279637. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Koehler D. E., Leonard R. T., Vanderwoude W. J., Linkins A. E., Lewis L. N. Association of latent cellulase activity with plasma membranes from kidney bean abscission zones. Plant Physiol. 1976 Sep;58(3):324–330. doi: 10.1104/pp.58.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miflin B. J. The location of nitrite reductase and other enzymes related to amino Acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974 Oct;54(4):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Hiraike K. Effects of centrifugal force and centrifugation time on the sedimentation of plant organelles. Plant Physiol. 1982 Feb;69(2):546–548. doi: 10.1104/pp.69.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P. Malic dehydrogenases in corn root tips. Arch Biochem Biophys. 1968 Jul;126(1):1–7. doi: 10.1016/0003-9861(68)90552-3. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]


