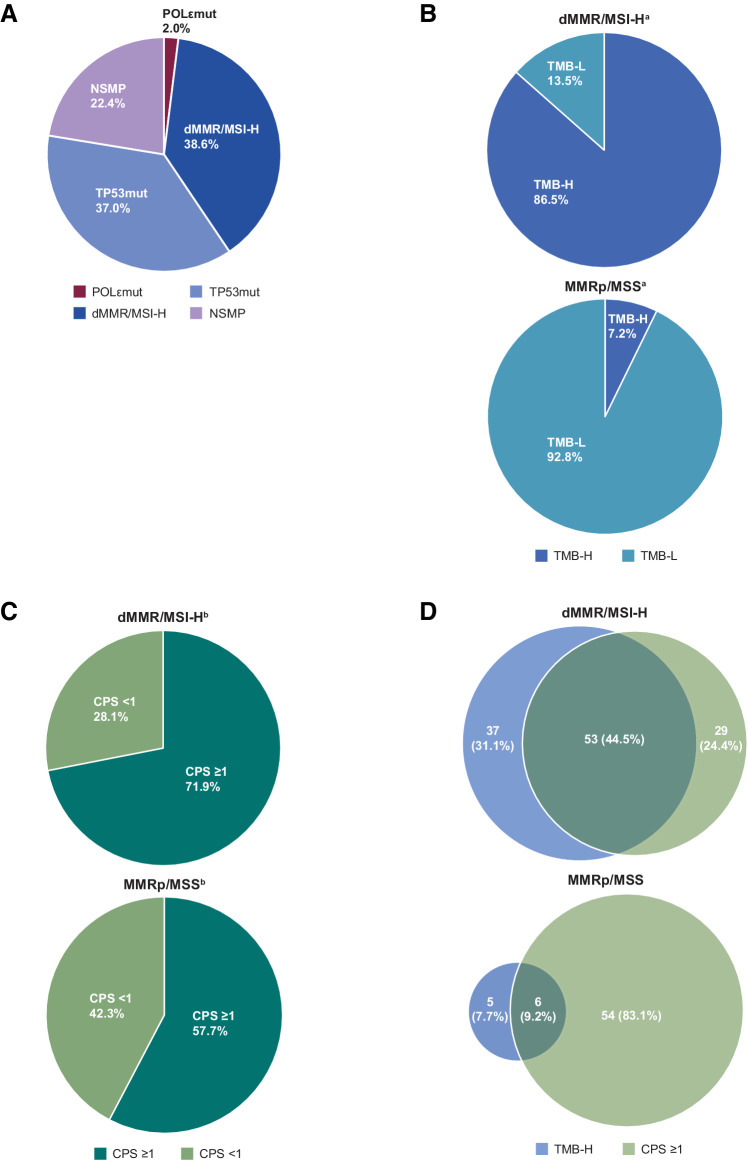
Figure 3.
Biomarker distribution in patients with advanced or recurrent EC treated with dostarlimab monotherapy. A, Percentage of EC tumors with molecular biomarkers in merged cohorts, including POLεmut, TP53mut, NSMP, or dMMR/MSI-H. B, TMB status of tumors in patients with dMMR/MSI-H EC (top) or patients with MMRp/MSS EC (bottom). C, CPS of tumors in patients with dMMR/MSI-H EC (top) or patients with MMRp/MSS EC (bottom). D, Venn diagrams showing percentages of tumors with TMB-H or CPS ≥1 or both in patients with dMMR/MSI-H EC (top) or patients with MMRp/MSS EC (bottom). aTMB status was available for 104 patients with dMMR/MSI-H tumors and 152 patients with MMRp/MSS tumors. bCPS status was available for 114 patients with dMMR/MSI-H tumors and 104 patients with MMRp/MSS tumors. TMB-NA, tumor mutational burden results were not available or undetermined.