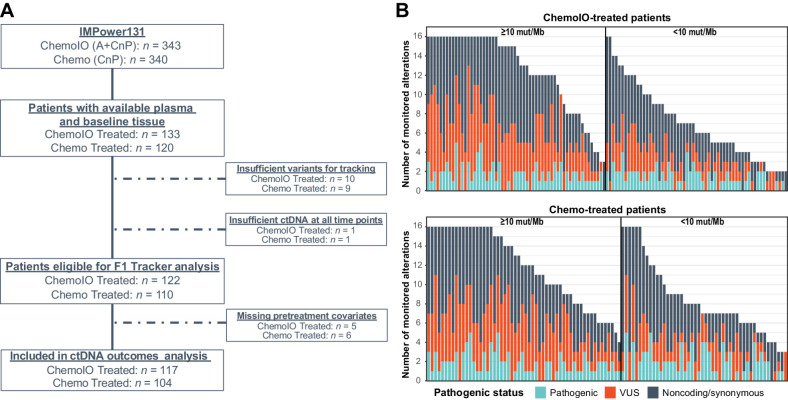
Figure 1.
Patient cohort for analysis. Per the CONSORT diagram (A), of 253 patients with tissue NGS results and plasma available, 232 (92%) were eligible for analysis using FoundationOne Tracker and 221 (87%) were included in ctDNA outcomes analysis. B, Distribution and number of monitorable variants per patient according to pathogenic status and TMB. Across the 221 patients with sufficient variants for FoundationOne Tracker, the median number of trackable variants was 13 in those with TMB ≥ 10 and 7 in those with TMB < 10. Chemo, chemotherapy; ChemoIO, chemotherapy plus immunotherapy; NGS, next-generation sequencing; VUS, variant of unknown significance.