Abstract
Background and Objective
The landscape of paediatric inflammatory bowel disease (pIBD) continues to evolve in an era of increasing incidence. There have been rapid developments in understanding, as we begin to perceive IBD as a spectrum of conditions, alongside advancements in monitoring and treatment. The objective of this article was to provide an overview of recent advances and challenges in the management of pIBD, with a focus on sustainable healthcare, personalised therapy, genomics, new drugs and avenues for future optimisation.
Methods
We present a narrative review that synthesises and summarises recent research (2017–2022) related to pIBD. We undertook a structured search of the literature (PubMed and Medline) and additional articles were identified through manual searches of reference lists. Evidence tables were compiled for disease outcomes.
Key Content and Findings
In this review we outline current practice, integrating clinical guidelines and contemporary research. We discuss initial investigations (including suggested threshold for paediatric faecal calprotectin), specialist investigations for disease monitoring [with reference to video capsule endoscopy (VCE) and therapeutic drug levels] and outline new and established treatment options. Biomarkers and genomic testing are examined as important tools for individualising care and identifying potential therapeutic targets, including for top-down therapy. Despite these advances, significant challenges remain, including the need for further research to understand the mechanisms of disease and the translation of these advances into real-world improvements in practice.
Conclusions
Recent advances in understanding of the pathogenesis of pIBD, alongside genomic and pharmacological developments have added more tools to the armamentarium for the treatment of these conditions and highlighted ongoing areas of research need.
Keywords: Paediatric, ulcerative colitis, Crohn’s disease, inflammatory bowel disease (IBD)
Introduction
The global incidence of paediatric inflammatory bowel disease (pIBD) has been steadily increasing over the past two decades, coinciding with advancements in the management of these diseases in children (1). Our understanding of IBD has evolved beyond Crohn’s disease, ulcerative colitis, and IBD unclassified, as we integrate macroscopic and microscopic findings from ileocolonoscopy with genetic and phenotypic disease behaviours (2). This integration has led to a significant breakthrough in our comprehension of monogenic causes of intestinal inflammation (3). Furthermore, the landscape of IBD has been transformed by the growing utilization of monoclonal and biologic therapy, proactive disease monitoring, and an increased number of diagnoses.
Parallel to this, developments in adult IBD, which encompass new therapeutic targets such as JAK-STAT inhibitors, IL-23 antagonists, and anti-integrins represent a widening array of treatment options for disease management (4,5). Although therapeutic options have expanded, and our understanding of the condition’s pathophysiology has improved, the translation of these advancements into improved short, medium, and long-term outcomes has progressed at a slower pace. Deep remission rates, despite extensive discussions, remain persistently low (6). The development of prediction algorithms to inform treatment decisions and improve outcomes, as well as the prevention of complications, has proven challenging and is not yet a routine practice. Additionally, the promise of genomic discoveries has yet to be fully realized in clinical settings.
However, there is emerging data indicating improved outcomes, including reduced surgical resection rates in childhood, increased use of steroid-sparing therapies, and better preservation of growth. With the increasing number of patients, the delivery of high-quality care in a safe and effective manner, while incorporating the latest evidence, continues to test clinical teams (2).
This narrative review aims to focus on the assessment and management of inflammatory bowel disease in childhood. It will discuss current outcomes and explore the potential translation of recent research findings into clinical practice. We have selectively included works published within the last 5 years (2017–2022) to emphasize recent evidence, and have focused on presenting these works from a UK and European perspective. Additionally, we will highlight the role of sustainable healthcare, personalized therapy, and improved access to treatments as important avenues for future optimization. We present this article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-210/rc).
Methods
A literature search was undertaken using electronic databases, utilising the strategy outlined in Table 1 to identify recent works relevant to recent developments in the assessment and management of pIBD—particularly within the UK and Europe.
Table 1. The search strategy summary employed for retrieval of relevant research works.
| Items | Specification |
|---|---|
| Date of search | 20/12/2022 |
| Databases and other sources searched | PubMed and Medline |
| Search terms used | Paediatric or pediatric inflammatory bowel disease or IBD or Crohn’s disease or CD or Ulcerative colitis or UC AND outcomes or surgery or remission rates OR Paediatric or pediatric inflammatory bowel disease or IBD or Crohn’s disease or CD or Ulcerative colitis or UC AND treatment OR management |
| Timeframe | 2017–2022 |
| Inclusion and exclusion criteria | Inclusion criteria: adequate study size, with necessary duration of follow up, studies focused on Europe and the United Kingdom, English language, retrospective and prospective studies containing outcome data of interest |
| Exclusion criteria: studies with inadequate sample size and/or follow-up, studies beyond area of interest, non-English language works | |
| Selection process | Green Z searched the databases for the search terms. All article types reporting data from retrospective and prospective studies were eligible for inclusion. Studies reporting data from Europe, and within the last 10 years were prioritised for inclusion. All studies were cross checked by Ashton JJ |
| Any additional considerations, if applicable | Review articles included to illustrate specific points |
Epidemiology, pathogenesis and increasing health burden
Incident rates of inflammatory bowel disease have been increasing globally within the paediatric population over the last two decades (1). The greatest increases in incidence have been observed in Western populations, including the UK, Europe and North America (7). Multiple causes have been suggested for increases in pIBD, including westernisation of diet, aseptic childhood, upbringing and genetic predisposition, although it is unlikely that such significant of a drift could have occurred over such a recent time period (8).
Support for dietary theories
Support for dietary theories has been achieved through epidemiological research, whereby rates of IBD amongst immigrant populations from lower-income countries to Western countries see a rise in incidence in IBD, hypothesised to be related to the adoption of a Western diet. The western diet often has high levels of animal protein and fat, with low intake of vegetables, fruit and fibre. Several mechanisms have been discussed for how this may translate into increased intestinal inflammation. One proposed model proposes that exposure to too great an amount of omega-6 polyunsaturated fatty acids (PUFAs), which are precursors to proinflammatory compounds and too few omega-3 PUFAs which inhibit the inflammatory process, may have a role in disease causation (9). Other proposed mechanisms implicate the high levels of additives and emulsifiers in the western diet and their effects on intestinal permeability (10). IBD pathogenesis is also consistently linked with dysbiosis within the commensal organisms of the gastrointestinal tract and the western diet is likely contributory to this (11).
Genetic and genomic factors in pathogenesis
The pathophysiology of IBD has considerable diversity and significantly differs between patients (5). The genetic architecture of IBD is similarly heterogenous, with multiple genes across immune regulation, inflammatory response pathways, and barrier function being implicated (12). The immune response seen in disease may be related to underlying genomic risk, coupled with environmental triggers (12). For some patients an inadequate immune response, related to hypomorphic variants in key bacterial sensing and signal transduction pathways—such as the NOD-signalling pathway—is the cause of disease. For other individuals, hyperinflammatory or autoinflammatory variation is the culprit (13,14).
Environmental triggers and the microbiome
The most implicated environmental trigger is intestinal bacteria, with a dysbiotic microbiome being associated with both disease onset and ongoing inflammation (15). However, the causal relationship between microbiome and the dysregulated immune response that characterises the ongoing inflammation within the gastrointestinal tract, remains unclear (16).
As the pathogenesis of IBD continues to be unravelled it is likely that molecular subtypes of disease will emerge, providing a more precise diagnosis for patients and allowing targeted management strategies to be developed (2). While the exact pathogenesis of IBD is unknown, a complex interaction between genetic, environmental and immune factors is implicated (17). As our understanding of the multiple streams of these contributory factors develops, we are increasingly able to identify IBD as a continuum, as opposed to specific conditions with multiple different phenotypes.
Implications
With numbers of diagnoses of pIBD increasing, escalating costs and pressures on services are expected. Increased staffing, facility for investigations and provision of biologic therapies are expected to represent a significant proportion of this burden. These costs will continue, on individual and population levels, following patients through their transition into adult care and throughout their lives (15,18).
Referral pathways and the use of faecal calprotectin
Initial presentation
There is considerable variability in how IBD is diagnosed and how services are accessed in the UK and Europe, with no common referral pathway for children and young people established. Patients may present via a variety of referral areas including general and specialist paediatric services as well as through general practice and allied healthcare practioners. Due to the heterogeneity of these diseases, as well as the broad age range of those presenting with IBD, symptoms can be varied and difficult to interpret.
National Institute for Health and Care Excellence (NICE) have published advice on initial steps in primary care. This document suggests that people with symptoms for greater than six weeks of abdominal pain or discomfort, bloating or change in bowel habit (such as diarrhoea with or without rectal bleeding) should be referred for faecal calprotectin or for further assessment (19). As aforementioned, children and young people with inflammatory bowel disease present with less characteristic symptoms—with only 25% of children going on to have a diagnosis of Crohn’s disease demonstrating the triad of abdominal pain, weight loss and diarrhoea at presentation (20). Prevailing symptoms may be more intangible such as lethargy, anorexia, altered abdominal sensation or discomfort and growth failure. This variable pattern of presentation can lead to delays in referral to specialist services, although NICE have set a goal for specialist assessment within 4 weeks of referral. This target is rarely achieved in the adult population and there has been an National Health Service (NHS) England statement advising local health authorities to establish and develop pathways to strive towards these targets (21).
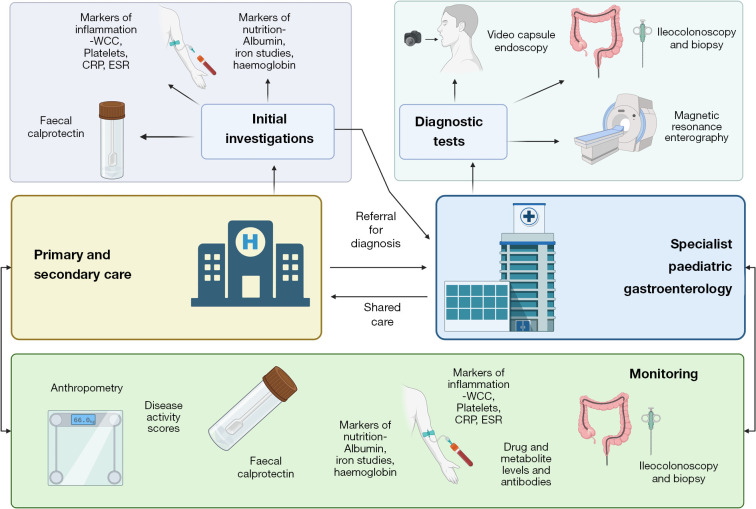
In practice, the difficulties in delineating symptoms, and relatively infrequency of presentation of children and young people with symptoms of IBD to non-specialist services, a combination of various markers is often requested. This often includes faecal calprotectin, blood markers of inflammation [C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white cell count (WCC)] and nutrition (iron studies, haemoglobin). It is important to note that normal bloods can occur in up to 10% of patients with new-onset IBD (22). As a result, faecal calprotectin remains an invaluable tool in highlighting children who require referral and may require endoscopy for suspected IBD. Figure 1 summarises a potential standardised diagnostic and management pathway.
Figure 1.
Summary of a potential diagnostic and referral pathway for paediatric-onset inflammatory bowel disease, including integration of monitoring and drug dosing between primary, secondary and specialist care. WCC, white cell count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Faecal calprotectin
Faecal calprotectin is a protein released from neutrophils, which are present in the gastrointestinal tract in areas of inflammation (23,24). Calprotectin, in children with chronic gut symptoms, can be utilised to categorise low risk of IBD (19). While in the adult population it is utilised to distinguish between IBD and irritable bowel syndrome (IBS), in children, NICE supports the use of calprotectin to differentiate between IBD and functional abdominal symptoms (25). It is also well established as a tool for monitoring inflammation and disease control once a formal diagnosis of IBD has been made.
Use of faecal calprotectin for initial assessment of the paediatric population remains controversial and access to this test is limited to secondary care in some health localities in England and Wales. Calprotectin has been demonstrated to have high sensitivity as a marker for gastrointestinal inflammation, however has low specificity (26). In addition to this there are few established reference ranges for normal or concerning values in the paediatric population, as exist in adult patients, though this is a particular area of ongoing research interest (24). Moreover, multiple factors may affect faecal calprotectin result, such as concurrent medication (i.e., ibuprofen); alternative diagnoses—for example, infection and polyps—and there is established variability within the individual throughout the course of the day. Younger patients, especially those aged <6 years, have higher calprotectin levels and results up to 500–600 µg/g should be interpreted with caution. There is also decline in overall calprotectin level within samples if there is a delay in analysis once obtained (27).
In the adult population a normal range is integrated into current practice at (<50 µg/g of stool) though various local pathways determine this level at different values ahead of progression to colonoscopy (28). Further work has suggested that a range of 50 to 250 (µg/g of stool) be considered “indeterminate”, wherein serial measurement may guide in selecting those that may later progress to ileocolonoscopy (29). British Society of Gastroenterology (BSG) guidance suggests that local referral to endoscopy should factor in local audit values for threshold value of calprotectin. They state that a balance must be found between higher thresholds, wherein fewer necessary tests will be performed, and some cases missed, and lower thresholds where fewer cases will go undetected, but unnecessary tests will be undertaken (30). BSG do not suggest that there is enough evidence to support repeat measurements, though in paediatrics, particularly in younger children where “normal” values may be higher or more variable, recent work has supported this (27).
Various threshold values have been suggested in paediatric practice, with values for monitoring suggested by European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) noting <100 µg/g usually reflecting remission and >250 µg/g predicting mucosal inflammation (31). A summary of recent work to determine threshold values for faecal calprotectin in the paediatric population is shown in Table 2 (24,27,32-36). In practice, further work is required to integrate calprotectin results with a combination of risk factors for IBD, whilst accounting for other influences on the result (i.e., age, gender, medication, concurrent pathology).
Table 2. A summary of primary data 2017–2022 evaluating faecal calprotectin in a healthy paediatric population including average results by age and suggested threshold value if stated (24,27,32-36).
| Study name | Year | Location | Patient group | No. of participants | Important inclusion or exclusion criteria | Average FCP (units specified) | Suggested threshold value |
|---|---|---|---|---|---|---|---|
| Fecal calprotectin level in healthy children aged less than 4 year in South Korea | 2017 | South Korea | Healthy children: <4 y | 234 | Excluded if positive stool culture, preterm birth | FCP by age, median [95th percentile] (μg/g): 7–12 mon, 78.5 [135]; 13–18 mon, 29 [65]; 19–24 mon, 27 [55]; 25–30 mon, 27 [40]; 31–36 mon, 12.5 [21]; 37–48 mon, 12 [12]; total, 24.5 [35] |
– |
| Paediatric reference ranges for faecal calprotectin: a UK study | 2017 | Bristol, UK | All faecal calprotectin results 2011–2014 |
Total: 8,673; <18 y: 723 | – | FCP, median [calculated] ULN (μg/g): 1–3.9 y, 49 [77]; 4–17.9 y, 40 [62]; 18 y, 56 [61] |
Proposed upper limit of normal of 77 for <4 |
| Comparison of fecal calprotectin in exclusively breastfed and formula or mixed fed infants in the first six months of life | 2017 | Tehran, Iran | Breast fed and formula/mixed fed infants | 60 | Significant antenatal, peri- or postnatal concerns |
FCP, mean (SD) (μg/g): 1 mon, breastfed exclusive, 368.85 (204.49), formula or mixed, 152.59 (139.13); 6 mon, breastfed exclusive, 283.21 (381.41), formula or mixed, 113.62 (92.75) |
– |
| Fecal calprotectin and eosinophil-derived neurotoxin in healthy children between 0 and 12 year | 2017 | Betera, Spain | Healthy children: 0–12 y | 174 | No concurrent established illnesses or medications known to increase faecal calprotectin | 95th percentile (μg/g): 0–12 mon, 910; 1–4 y, 286; 4–12 y, 54 |
– |
| Fecal calprotectin in healthy children aged 4–16 year | 2020 | Betera, Spain | Healthy children: 4–16 y; median: 9.2 y | 212 | No concurrent established illnesses or medications known to increase faecal calprotectin | Median (95th percentile) (mg/kg): 4 y, 24.8 (187.4); 16 y, 14.4 (60.7); total, 18.8 (104.5) |
– |
| Normal fecal calprotectin levels in healthy children are higher than in adults and decrease with age | 2020 | Madrid, Spain | Healthy volunteers: 0–18 y | 395 | No concurrent established illnesses or medications known to increase faecal calprotectin | Median [IQR] (μg/g): 0–1 month, 303 [202]; 1–5 mon, 325.5 [375]; 6–11 mon, 63 [126]; 12–23 mon, 97 [275]; 2–3 y, 71 [130]; 4–7 y, 46 [89]; 8–11 y, 34.5 [48]; 12–18 y, 30 [19]; total, 77 [246] |
>250 μg/g |
| Guidance on the interpretation of faecal calprotectin levels in children | 2021 | Cambridge, UK | Children: <16 y | All calprotectin: 2,788; referred to specialist: 373 | – | Median [IQR] (μg/g): <1 y, 205 [498]; 1–5 y, 55 [120]; 6–14 y, 41 [80]; 15–16 y, 47 [113] |
>250 μg/kg with other positive biomarkers |
FCP, faecal calprotectin; ULN, upper limit of normal; y, years; mon, months; SD, standard deviation; IQR, interquartile range.
Developments in specialist investigations
Endoscopy
The primary investigation for suspected IBD following referral to specialist paediatric gastroenterology services is endoscopy, typically under a general anaesthetic or with sedation for older children. Diagnosis of IBD must be made through upper and lower gastrointestinal endoscopy, in line with porto group recommendations (37). The diagnosis of IBD is clinicopathological, integrating histology with macroscopic appearances, and small bowel imaging. It is the consideration of these factors together which may also aid with differentiation between disease subtypes and subsequent management decisions. While the technical capabilities of endoscopic equipment have advanced over recent years, the manner in which these tests are employed have deviated very little from the ESPGHAN position paper on this topic (38). Contemporary discourse focuses on non-invasive—or less-invasive—imaging techniques for the diagnosis and monitoring of inflammation. This includes the refinement and evaluation of existing, established modalities such as ultrasound and magnetic resonance imaging (MRI), in addition to direct visualisation through capsule endoscopy, the use of which is rising in UK and European centres (39).
Small bowel imaging and video capsule endoscopy (VCE)
The ESPGHAN revised porto criteria recommend small bowel imaging for all children with a suspected diagnosis of IBD, unless ileocolonoscopic and histological features are in keeping with typical ulcerative colitis in which case this can be deferred (37). MRI or magnetic resonance enterography (MRE)—MRI with oral contrast—are usually utilised for this purpose and also for the capture of the sequelae of IBD i.e., fistulating or stricturing disease and extra-intestinal disease (40). MRE, however, is limited in sensitivity for the detection of very subtle mucosal changes (37). As a result, there is drive to achieve direct small bowel visualisation, for which VCE is increasingly employed.
VCE has been demonstrated to provide superior detection of superficial small bowel mucosal inflammation in Crohn’s disease over MRE, with otherwise comparative rates of localisation of inflammation between modalities (41). Capsule endoscopy has been demonstrated to have superiority in detecting small bowel involvement in Crohn’s disease over small bowel radiography, computed tomography (CT) and colonoscopy and ileoscopy in a meta-analysis in adult patients, and can be achieved with minimal discomfort (42).
Owing to these advantages, VCE is increasingly utilised in the paediatric population for visualisation of the small bowel mucosa; which cannot be visualised through conventional endoscopy or after inconclusive findings in MRE (43).
Challenges of VCE
In spite of promising contemporary work, the implementation of VCE can prove challenging in the paediatric population. These challenges include capsule retention, the need for bowel preparation and difficulties with swallowing the capsule necessitating endoscopic placement (39,43). The greatest risk factor for capsule retention is known IBD (5.2%) and risk increases drastically when combined with a body mass index (BMI) under the fifth percentile (43%) (44). With the aim of mitigating the risk of retention, patency capsules have been developed in the same dimensions as the video capsule. Although retention remains a risk with these techniques, no perforations or obstructions have been reported in the paediatric population.
VCE is also limited by the inability to affect the movement of the capsule during transit, and the lower specificity of the test, which can lead to increased detection of incidental findings (37).
Contraindications to VCE
Various contraindications have been described by the manufacturers of video capsules and in contemporary research. These include known or suspected obstruction, intestinal stricture, previous abdominal surgery (relative) and use in children under one year. In addition to this, motility and swallowing disorders are also included as contraindications in some manufacturer’s information (45). While intestinal dysmotility remains a barrier to VCE, and can be evaluated with patency capsule, milder motility issues can occasionally be overcome with prokinetic medications (43). Moreover, as aforementioned, the direct introduction of the video capsule into the duodenum via endoscopy can be utilised to ameliorate dysfunctional swallow (37).
Future developments in VCE
Despite these areas of concern regarding the usage of VCE, direct visualisation of the small bowel is increasingly necessary. With STRIDE and STRIDE-II as well as ESPGHAN and European Crohn’s Colitis Organization (ECCO) guidance targeting histological and mucosal healing as targets for treatment, direct visualisation is required to guide management (46,47). Moreover, accurately detecting small bowel inflammation in Crohn’s disease is particularly important, for when this is left uncontrolled it can lead to arrests in growth and nutrition and therefore significant morbidity (48). The level of direct visualisation offered by VCE can be a useful addition to existing tools for initial investigation and monitoring of IBD, providing greater appreciation of small bowel mucosa than MRE, however the modality is limited by the lack of functionality for collection of tissue samples. In spite of this, the continued miniaturisation and improvement on ingestible electronic technologies, with upgrading in visual quality through higher frame rates and the integration of additional sensors and microrobotics, makes VCE increasingly viable for use in the paediatric population for direct visualisation of the small bowel (49).
Treatment options and strategies
The management of IBD is multi-faceted and requires multidisciplinary team input. The general principles, utilised in the UK and Europe, are set out in position papers from the ESPGHAN-ECCO groups, alongside more specific guidelines related to management in paediatric and adult cases produced by the BSG, and centre on induction of remission with long-term mucosal, or transmural healing (30,31,50,51). Management of symptoms, psychological aspects of care and paediatric specific considerations, such as growth and schooling must be carefully considered.
Nutritional therapies
Exclusive enteral nutrition (EEN)
As previously discussed, the pathogenesis of IBD likely incorporates environmental and dietary exposure factors with genetic factors in leading to disease phenotype. As a result, altering these factors through dietary manipulation is a target for treatment. Chief amongst these treatments is EEN (52). This involves the delivery of a formula feed, providing complete nutritional balance either orally or via enteral feeding tube (53). Formulas can be defined by their protein source i.e., complete proteins (polymetric), modified (elemental) or they can be disease-specific.
EEN is widely used as primary therapy for induction of remission in paediatric Crohn’s disease and is recommended in ECCO and ESPGHAN consensus statements for mild and moderate uncomplicated disease. These statements, integrating several systematic reviews, have defined an overall combined remission rate of 73% for EEN, noting similar efficacy for this purpose as corticosteroids (47,54). In addition to this, greater levels of mucosal healing have been noted, as well as the correction of nutritional deficiencies, improvements in lean body mass and quality of life, all with the benefit of sparing steroids and their avoiding their sequelae (53,55,56). These benefits however are limited to patients with luminal Crohn’s disease, as there are insufficient data to support the use of EEN for extraintestinal and perianal involvement, or in ulcerative colitis (54).
While palatability and tolerance of EEN stand out as the main disadvantages of the therapy, strong supportive evidence for induction of remission, in addition to several benefits over induction therapy with steroid medications, make EEN one of the most useful tools available to the paediatric gastroenterologist (53,55,56).
Partial enteral nutrition (PEN)
In overcoming the limitations of palatability and tolerance in EEN, some treatment strategies have utilised formula-based enteral nutrition alongside normal diet, with varying amounts of formula feed given (52). Historically, PEN has not demonstrated to be as efficacious as EEN for treatment of active disease, particularly when a normal diet is permitted alongside a specified volume of feed (57). A recent meta-analysis of work looking at PEN versus EEN has been published which has demonstrated similar response rates, however these results rely on restrictions to what diet is permitted, with the greater the imposition on normal diet, the greater the effect (58). As a result of this PEN has a lesser role in the induction of remission in this group.
Crohn’s disease treatment with eating diet (CD-TREAT)
The CD-TREAT diet aims to mimic the composition of EEN, whilst integrating whole foods to overcome issues with palatability (59). CD-TREAT is a prescriptive and personalised diet designed to best emulate the constituent levels of protein and carbohydrate in EEN, with the exclusion of some components such as gluten, lactose and alcohol (60). Initial work has been mostly positive, with higher levels of tolerability in the adult population, similar microbial, clinical and inflammatory improvements as seen with EEN, however study populations in paediatrics have been small and further work is required in this area.
Crohn’s disease exclusion diet (CDED)
The CDED is a whole food-based diet that aims to remove foodstuffs that are implicated in gastrointestinal dysbiosis or inflammation (59). In practice this involves the combination of PEN with specific exclusions. Contemporary research work has noted comparable induction of remission rates in children allocated CDED, when compared with EEN at up to 80% (61,62). In these works, the quantity of PEN was gradually weaned with increase in whole food content in diet, with remission largely being maintained. Tolerability in these groups was superior to EEN. With this dietary regime showing early promise, further data are required before CDED can be recommended routinely in the management of CD (54).
Other diets
Several other exclusion diets have been reported for the management of inflammatory bowel disease. These include the Specific Carbohydrate diet, the Low in Fermentable Oligo-, Di- and Monosaccharides and Polyol (FODMAP) diet, and exclusionary diets such as gluten-free and vegan. Other works have reported on the exclusion of foods to which the participant has a high IgG4 titre response (54,56). There is not, however, sufficient evidence for the recommendation of these dietary regimes, and the risk of dietary restriction and malnutrition may outweigh significant benefits.
Medical agents
Corticosteroids
Induction
The mainstay of induction therapy in ulcerative colitis is corticosteroid therapy, which may be intravenous in more severe disease, or oral in moderate inflammation (63). A slow tapering of steroids over a 6 to 10-week period is required, during which time maintenance agents should be introduced (63). Steroids are efficacious for induction of remission, with rates of 50–64% reported, which is comparable to EEN (63). Topical steroids may be utilised in some situations, with corticosteroid enemas given as a longer-term treatment strategy in isolated or difficult to control proctitis. However, his is not commonly utilised in paediatric practice (63,64).
In Crohn’s disease, steroids are a highly effective treatment, and again may be oral of intravenous depending on disease severity (51). Modified-release steroids are also available for isolated mild terminal ileitis and can be a highly effective induction strategy in these patients (51).
Maintenance
The side effect profile of corticosteroids, particularly in prolonged and “supraphysiological” dosing is well documented, with weight gain, cushingoid features, osteoporosis, glaucoma, hypertension and mood disturbance being commonly seen (65). As a result, after weaning, steroid sparing strategies are favoured for the maintenance of remission to avoid these side effects, which can be particularly significant in a paediatric population—i.e., bringing about growth and pubertal delay (63).
5-aminosalicylates (5-ASAs)
Induction
In mild ulcerative colitis, 5-ASAs can be used as an induction treatment, although the response to treatment must be closely monitored to ensure adequate and timely remission. The majority of paediatric patients with mild-moderate ulcerative colitis will not have a response with oral 5-ASA alone, with rates of 35–55% noted (63). Meta-analysis has demonstrated that there are not sufficient data to support the use of 5-ASA in Crohn’s disease (66).
Maintenance
In mild ulcerative colitis, or IBD unclassified with mild colitis, there is a role for monotherapy with 5-ASA. Until recently, evidence suggested that 5-ASA should be used in combination with other treatments for ulcerative colitis potentially as a preventative measure against development of neoplasia (63). However, recent adult data from over 8,000 patients did not find any additional benefit from 5-ASA when an immunomodulator or anti-tumour necrosis factor (anti-TNF) therapy was concurrent (67).
Immunomodulators
Induction
There are insufficient data to support the use of immunomodulating drugs, i.e., thiopurines and methotrexate, for the induction of remission in inflammatory bowel disease (68).
Maintenance
Immunomodulators continue be highly prevalent in the maintenance of remission. Within the United Kingdom, thiopurines including azathioprine and 6-mercaptopruine, are used with more frequency than methotrexate, although both demonstrate efficacy (69,70). Increasingly we are finding genetic predictors of response and toxicity for thiopurine medications, such as those within the TPMT and NUDT15 pathways, which will provide significant patient benefit and risk minimisation as pharmacogenomics moves into the mainstream (71,72). Ongoing concerns regarding lymphoma risk, especially in Epstein-Barr virus-naïve male patients, have caused reductions in prescribing in some areas, although the absolute risk remains extremely small (51,73,74).
Immunomodulators also have a role in prevention of immunogenicity against anti-TNF therapy, and may frequently be used in combination with monoclonal therapy in the initial phase. There is a move to withdrawal of concurrent immunomodulation after 1 year leaving anti-TNF monotherapy, although subsequent long-term combination therapy continuation remains commonplace (75).
Anti-TNF therapy
Induction
Anti-TNF therapy, primarily infliximab—monoclonal chimeric anti-TNF antibody—and adalimumab—a fully humanised monoclonal anti-TNF antibody, have become mainstays of paediatric treatment (76). Increasingly data are pointing towards early introduction of therapy with these medicines—a “top-down approach”—as being highly beneficial for some patients (77). Furthermore, use of biologics should be expedited in cases of fistulating Crohn’s disease, perianal disease and where significant small bowel disease is present (51).
The most notable recommendations for anti-TNF therapy comes for moderate-severe Crohn’s disease, with the most recent ECCO-ESPGHAN guidelines recommending this as first line therapy (51). Similarly in severe ulcerative colitis, most commonly in the presence of acute severe colitis, there may be the need for primary induction with anti-TNF therapy (most commonly infliximab), alongside corticosteroids. Proactive drug monitoring in the initial induction phase is increasingly driving tailored dosing regimen after the 2nd, 3rd or 4th infusion, although clinically there is still equipoise on the long-term benefit from this strategy (78).
Maintenance
The role of anti-TNF therapy in pIBD continues to expand for use in disease maintenance. Not all individuals will require these medicines however, and not all will respond. Primary non-response (PNR) rates remain high at up to 30%, with an additional 25–50% subsequently losing response (79,80).
Dosing of anti-TNF therapy has progressed, and tailoring dose, dosing interval and concurrent therapy to the patient’s need should be routine. Drug monitoring is explored in greater detail within the Therapeutic monitoring section of this article. Further insights into genomic drivers of response, or loss-of-response are also emerging, and this may result in personalised pharmacogenomic profiling, allowing the correct drug to be selected for the molecular phenotype, prevention of immunogenicity or toxicity and stratified clinical trials (75). Therapeutic drug monitoring (TDM) in anti-TNF therapy has become a mainstay of management with these medications. The evidence on proactive vs reactive drug monitoring remains conflicted (81). Despite this, there are many unmet problems in optimising use of biological therapy including identification of the optimal drug concentration (for specific patients or subtypes), the impact of point-of-care TDM and the interpretation of anti-drug antibody titres (82).
New generation’ monoclonal therapy and small molecule drugs
Induction
Currently there is no evidence to suggest that induction with alternative biological or small molecule therapies has a role in Crohn’s disease or ulcerative colitis in children (51). Despite this, data from adult studies would suggest that JAK-STAT inhibitors can be highly effective at induction in ulcerative colitis and some data from paediatric practice is observing highly effective rescue therapy in acute severe colitis (83,84).
Maintenance
Monoclonal agents
Increasingly paediatric gastroenterologists are turning to newer monoclonal therapies for patients who have do not respond, or lose response, to anti-TNF therapy. The main classes are the anti-α4β7 integrin, vedolizumab, and the anti-IL-23 therapies including joint anti-IL-12/23 drugs, such as ustekinumab, and targeted anti-IL-23 drugs, such as risankizumab.
Primarily, vedolizumab is used in colonic disease, as its mechanism of action prevents chemotaxis of immune cells into the large bowel, and it thus more suited to ulcerative colitis. Ustekinumab, originally licensed as a therapeutic agent for psoriasis, is used more in Crohn’s disease, as is the newer and more specific, risankizumab. All therapies have shown effectiveness in treatment-refractory paediatric disease, although remission rates appear to vary between 44–76% depending on patient characteristics and drug choice (85,86). Increasingly, adult practice is to opt for monoclonals other than anti-TNF as first line, and this may become more apparent in paediatric disease as we develop tools to aid with therapeutic prediction and selection (87,88).
Small molecule drugs
Another contemporary, and exciting, group of therapies coming to paediatric practice are the oral small molecule drugs. Targeting alternative inflammatory pathways including JAK-STAT signalling (such as tofacitinib, upadacitinib) and sphingosine-1-phosphate receptor agonists (ozanimod). These newer therapies offer another avenue of treatment for patients. They are not yet licensed for paediatric practice but trial data from adult populations suggests relatively high efficacy in ulcerative colitis, with up to 60% clinical response in the maintenance phase of the trials (83,89,90). Promising efficacy data have been demonstrated with tofacitinib for induction and maintenance of remission in ulcerative colitis, particularly in the adult population, however certain concerns regarding the safety profile, including risks of infections and venous thromboembolism must be considered (91). Upadacitinib has also been shown to be effective for this purpose, with fewer safety concerns (90). Few case reports have utilised upadacitinib in the paediatric population, though contemporary work has noted efficacy and safety (92). Whether further support for the integration of these drugs into paediatric practice will emerge is yet to be seen, and options for Crohn’s disease remain more limited.
Key topics and practice points in disease management
Acute severe colitis
Acute severe colitis is one of few emergencies in paediatric gastroenterology, and without timely management can lead to significant morbidity and mortality (93). Guidance for management has been published by the European Crohn’s and Colitis Organisation (ECCO) in conjunction with ESPGHAN (93).
This work emphasizes the significance of excluding alternative and infectious causes of colitis, such as Clostridium difficile and cytomegalovirus (CMV), by conducting stool culture and mucosal biopsy. It is recommended to administer antibiotic and antiviral treatment when these organisms are detected. Additionally, it is important to consistently consider toxic megacolon or bowel perforation as potential complications, and to request an abdominal X-ray with worsening pain (93).
Prompt initiation of treatment is essential, and this typically involves administering intravenous (IV) methylprednisolone or an equivalent corticosteroid dose. It is advisable to regularly calculate paediatric ulcerative colitis activity index (PUCAI) scores, and if the score exceeds 45 on day three, preparations should be made for second-line management options such as cyclosporin, tacrolimus, or infliximab. If not already performed, sigmoidoscopy is recommended in such cases. In the paediatric population, infliximab is the preferred second-line therapy. Due to the potential rapid clearance of infliximab during acute severe colitis, higher dosing regimens may be utilized, and treatment should be guided by drug levels. If a single second-line agent fails, referral for colectomy is recommended. According to ECCO/ESPGHAN guidance, in specialized centres, after tapering off concomitant corticosteroid therapy, a third-line agent (either a calcineurin inhibitor after infliximab or vice versa) can be considered for trial (93). Thromboprophylaxis should also be considered concurrently (94).
Going forward
There is a growing body of work, within adult practice, supporting the use of small molecule drugs (particularly tofacitinib) for acute colitis (4,83,84,95). Early reports in children mirror these findings, with Constant et al., reporting 8 of 11 children with acute severe colitis (ASC) being colectomy free at 90-day post tofacitinib initiation and 6 of 11 remaining so at a median 182-day follow-up (96). Further work exploring the use of JAK-inhibitors is required in the paediatric population to support integration of these medicines into standard care. As use of newer agents emerges, it remains vital to include early involvement of the surgical team for consideration of colectomy.
Loss of response (LOR) to therapy and TDM
PNR or LOR, particularly to anti-TNF agents (infliximab, adalimumab) pose significant challenges for management of IBD (97). A multimodal cause for LOR or PNR is proposed, integrating a combination of genetic and environmental factors. A large prospective cohort study, undertaken by Kennedy et al., utilising anti-TNF agents, reported PNR occurring in 23.8%, and non-remission in 63.1%. They suggested that low drug concentration at induction, as well as several factors including smoking, low albumin, obesity, development of immunogenicity and higher disease score were predictive of non-response (98).
TDM, is employed to ameliorate PNR or LOR, and involves the measurement and interpretation of anti-TNF anti-drug antibodies (ADABs) in order to tailor dosing schedule and regime. If the drug level is below the desired target, in the absence of negative ADABs then escalation of dosing regime is suggested (99). If there is no improvement with higher trough levels, or antibodies present then class switching to, for example, the anti-integrin antibody medications is suggested (100).
There is ongoing debate on the benefits of reactive TDM, performing monitoring in response to the clinical situation, versus proactive drug monitoring, routinely monitoring drug levels and antibodies regardless of the clinical situation. The highest quality data can be extrapolated from adult practice although it is important to recognise there may be pharmacodynamic differences between children and adults. In 2022, there were two meta-analyses comparing proactive vs reactive TDM in anti-TNF therapy, resulting in different conclusions; Nguyen et al. found no clinical benefit when considering only 9 high quality randomised control trials (RCTs) (81), however significant benefits (reducing treatment failure, surgical rates and improved endoscopic remission and response) where reported by Sethi et al., who considered an additional 17 non-RCT studies (101).
Typically, TDM refers to biologic therapies, such as infliximab and adalimumab. Thiopurines can also be monitored and optimised, utilising 6-thioguanine nucleotide and 6-methylmercaptopurine metabolites. In this way, it is possible target therapeutic doses of azathioprine and 6-mercaptopurine, whilst avoiding toxicity (74). A growing body of work supports the use of combination therapy—i.e., employing these immunomodulating therapies such as 6-mercaptopurine, as well as azathioprine or methotrexate alongside anti-TNF agents—to ameliorate the immunogenic response to prolonged monoclonal therapy (102).
Data supporting therapeutic monitoring with vedolizumab and ustekinumab are more lacking, although adult studies have demonstrated improved mucosal healing with higher trough levels (103). Further work is required, particularly in a paediatric population, to support routine monitoring and the development of threshold values for this purpose (5).
Genetic polymorphisms, particularly in NFkB, IL-18, TNF-alpha and IL-1 Beta have been implicated in non-response to anti-TNF therapy and further data are required to explore how these can be combined into predictive modelling to tailor and individualise best treatment (104). Integration of genetic, clinical and risk factor data is required on an individualised basis to prevent medication exhaustion. In the future, there may be the potential shift to utilisation of point-of-care testing for patients, allowing for real-time alterations in drug dosing based on levels and anti-drug antibodies at the bedside, although whether there are clinical benefits remains unknown (105).
Personalised and precision therapy
With vast heterogeneity in pathogenesis, treatment responses and outcomes in pIBD, there is cause to move towards a personalised approach to diagnosis and to management (2). In this way, the integration of genomic, biomarker and environmental data from an individual could provide them an optimised and specific diagnosis, and bring about targeted prevention and treatment of disease (2). For IBD, this personalised or precision medicine, could equate to improved understanding and prediction for an individual patient, allowing for the right therapy to be given at the right time, avoiding complications and side-effects, whilst maximising therapeutic benefits. Strategies to predict outcomes based on molecular diagnostics and clinical data are beginning to emerge and are likely to break into the clinical sphere (106). Continuing to push forward this strategy will reduce medication failure, rationalise investigation and overall provide patient benefit (107).
Genetics in the management of IBD
Screening for single-gene causes of disease in IBD is now a standard of care for selected individuals—those aged <2 years at diagnosis, or those aged between 2–6 years of age with specific additional features associated with disease (108). In older patients, or patients presenting with no specific features of monogenic IBD there are emerging roles for genetics in the prediction of complications such as stricturing disease, in pharmacogenetics (such as for thiopurines and anti-TNF therapy) and as potential avenues for improving the molecular phenotype of disease to predict and guide therapy for individual patients (14,72,75,106). Translation of research findings to clinical benefit is beginning and will change the landscape of disease management within the next 10 years.
Disease outcomes
Is growth still an issue in paediatric Crohn’s disease?
Historical data has long indicated growth problems in paediatric-onset Crohn’s disease. Typically, this consisted of a mean height standard deviation score (SDS) for cohort being lower, at around SDS −0.3 (109,110). Large national cohorts looking at patients diagnosed between 1990–2014 confirm a long-term height deficit persisting into adulthood, although contemporary data from the Wessex pIBD cohort do not demonstrate a growth deficit during follow-up (111,112). Patients, however, continue to present underweight, with reported mean weight SDS values ranging from −0.65 to −1.0 (109,111). Despite this initial deficit there is a normal distribution of weight SDS by 1 year after diagnosis, although patients with more severe disease may still have growth issues (111).
It may be that prompt diagnosis, introduction of newer and more effective therapy, alongside improved nutritional care, is beginning to change the natural history of disease (113,114).
Complications of Crohn’s disease—stricturing and fistulating disease
Crohn’s disease behaviour is heterogenous, with phenotypes recognised as per the Paris modification of the of the Montreal classification of IBD (115). An estimated 75% patients present with inflammatory disease (Paris classification B1) and up to 25% of paediatric patients present with B2 (stricturing) disease (109,116). Typically, there is disease evolution throughout childhood and into adulthood, and by 10 years disease behaviour changes to stricturing disease (B2) in up to 50% of patients, fistulating disease (B3) in 5–35% patients, or occasionally both in 5% (116-118). There does not appear to have been a significant shift in the progression to complicated disease behaviour over the last 30 years, although there are demonstrable reductions in hospitalisations and additional measures of morbidity (119).
The change from a purely inflammatory B1 phenotype of Crohn’s disease over time presents additional management and surgical challenges. Intestinal resection rates in paediatric disease do appear to be reducing, with resection rates (primarily right hemicolectomy) reducing from 8.9% of patients in 1997 to 1.5% of patients in 2017 (120). National data reflects a similar picture with a decrease in resections identified since 2009 (121). Despite this, the long-term outcomes of patients with Crohn’s disease do not appear to have changed much, and it may be that resections are being delayed until after transition to adult services (122). There are ongoing high rates of perianal complications (up to 50%), surgical resections (up to 60%) and increased biological therapy use (up to 75%) by 10 years following diagnosis (123).
The impact of new therapies may not yet have been fully manifested. There appears to be a concurrent drop in intestinal resections with increased anti-TNF prevalence (120). Despite this, long-term outcomes of patients who withdraw from anti-TNF therapy appear to indicate that at 10 years approximately 50% will remain in remission on immunomodulators alone (124). An area where there is less controversy is the positive impact of anti-TNF therapy on perianal and fistulating disease, where there is excellent evidence to support early introduction to promote healing (51). The literature supports this position with data indicating top-down therapy being successful at resolving fistulae in 58–100% of patients at 12 months post-diagnosis (125).
Complications in ulcerative colitis
Ulcerative colitis does not have the wide range of potential complications seen in Crohn’s disease, but the condition remains highly heterogenous. Presentation with acute severe ulcerative colitis (ASUC) is a key complication for paediatric patients, with data indicating up to 15% of patients present with severe disease and during childhood 28–40% of patients will experience a severe exacerbation, although this may not always meet criteria for ASUC, associated with a paediatric ulcerative colitis activity index score of >65 (126). Steroid-refractory ASUC is seen in up to 1/3 of patients and requires escalation to additional therapy, such as high dose anti-TNF (126).
The need for surgery in paediatric ulcerative colitis has remained stubbornly high, although continues to be lower than for Crohn’s disease (121). Time series data from 1997–2017, and hospital episode statistic data over the same period, does not demonstrate a significant reduction in subtotal colectomy, performed for any reason, over the time-period, with incidence of resections varying between 0–5% per year (120,121). Single-centre cohorts continue to point to the incidence of colectomy being around 10% during childhood (127).
There is a relative paucity of long-term outcome data for liver disease (autoimmune sclerosing cholangitis and primary sclerosing cholangitis) in paediatric onset IBD, although the limited reports indicate that cirrhosis and liver transplantation are relatively common (12–30%) (128). Outcomes are summarised in Table 3.
Table 3. Long-term outcomes of paediatric inflammatory bowel disease—specific situations and key studies (2017–2022) (6,116,129-139).
| Study title | Year | Specific consideration and location of study | Disease subtypes | Population size, n | Follow-up duration | Remission rate at maximal follow-up | Intra-abdominal surgery rates | Perianal surgery/abscess rates | Structuring rate (B2 disease) | Fistulae rate (B3 disease) |
|---|---|---|---|---|---|---|---|---|---|---|
| Risk of colectomy in patients with pediatric-onset ulcerative colitis | 2017 | Pediatric ulcerative colitis; Schneider Children’s Medical Centre of Israel | Ulcerative colitis | 188 | Mean 6.9 years | – | 18% colectomy | – | – | – |
| Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study | 2017 | Paediatric Crohn’s disease; 28 sites in the USA and Canada | Crohn’s disease | 913 | 3 years | – | – | – | 54 (5.9%) | 24 (2.6%) |
| The characteristics and long-term outcomes of pediatric Crohn’s disease patients with perianal disease | 2017 | Pediatric Crohn’s disease—perianal disease; Schneider Children’s Medical Centre, Israel | Crohn’s disease | Crohn’s disease 296; 110 (37% perianal at diagnosis); 70 (24% with non-fistulating perianal disease) | Mean 8.5 years | – | 26% | 16% | – | – |
| Outcomes of a national cohort of children with acute severe ulcerative colitis | 2018 | Outcomes after acute severe colitis; Our Lady’s Children’s Hospital, Crumlin, Dublin | Ulcerative colitis | 55 | Mean 29 months | 53% | 38% | – | – | – |
| The long-term predictive properties of the Paris classification in paediatric inflammatory bowel disease patients | 2018 | Disease flare, hospitalisations, step-up to biologic treatment and surgical procedures | Crohn’s disease, ulcerative colitis | 427 total (301 Crohn’s disease, 126 ulcerative colitis) | Crohn’s disease: 9.1 years, ulcerative colitis: 8.5 years | – | Crohn’s disease: 4%, colectomy for ulcerative colitis: 18% | 16% in Crohn’s disease | 42.4% (B2–3 disease underwent surgery) | – |
| Long-term outcomes of paediatric patients admitted for acute severe colitis - a multicentre study from the paediatric IBD porto group of ESPGHAN | 2019 | Outcomes after acute severe colitis; 25 pediatric gastroenterology centers across Europe and North America | Ulcerative colitis | 141 | 5 years | – | 36.40% | – | – | – |
| Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study | 2019 | Pediatric ulcerative colitis; 29 centres in USA and Canada | Ulcerative colitis | 467 | 1 year | 38% | 5% | – | – | – |
| Progression to colectomy in the era of biologics: a single center experience with pediatric ulcerative colitis | 2020 | Paediatric ulcerative colitis; Texas Children’s Hospital | Ulcerative colitis | 217 | Mean ± SD: 5.02 (±2.27 years) | – | 12.9% colectomy | – | – | – |
| Clinical and host biological factors predict colectomy risk in children newly diagnosed with ulcerative colitis | 2022 | Pediatric ulcerative colitis; 29 centres in USA and Canada | Ulcerative colitis | 359 at 2 years, 269 at 3 years | –, – | – | 13% at 3 years, 9% at 2 years | – | – | – |
| Disease activity patterns in the first 5 years after diagnosis in children with ulcerative colitis: a population-based study | 2021 | Pediatric ulcerative colitis; SIGENP IBD group, Italy | Ulcerative colitis | 226 | 5 years | 24% disease extension, 13% ≥1 episode acute severe colitis | 8% colectomy | – | – | – |
| Clinical characteristics and long-term outcomes of pediatric ulcerative colitis: a single-center experience in Korea | 2022 | Paediatric ulcerative colitis; South Korea | Ulcerative colitis | 208 | 6.5 years | – | 8.7% colectomy | – | – | – |
| New therapeutic strategies have changed the natural history of pediatric Crohn’s disease: a two-decade population-based study | 2022 | Paediatric Crohn’s disease; EPIMAD registry, Northern France | Crohn’s disease | 580 in developing biologic era (2001–2011) | Median 8.8 years | – | 21.70% | – | 19.8% progressed to stricturing disease | 7.6% progressed to penetrating disease |
| Long-term follow-up and predictors of complicated disease behavior in pediatric Crohn’s disease patients | 2022 | Paediatric Crohn’s disease; Kaplan Medical Centre, Israel | Crohn’s disease | 93 | Median 10.3 years | – | 21.50% | – | 29.4% of those with B1 disease at diagnosis evolved to B2 and or B3 | – |
SD, standard deviation.
Additional outcomes to consider
Measuring pain and mental health outcomes has been largely ignored in clinical trials but remains a central feature of disease burden for many patients. Addressing these measurables in future studies will be important to reduce overall disease burden.
Adult data and impact on paediatric practice
Transition care
Transition services provided in conjunction with adult gastroenterology can be bidirectional learning opportunities for clinicians and patients. It is established that engaging and proactive transition to transfer patients from paediatric to adult services should begin early and be conducted in a structured format (140,141). Extensive guidelines for transitional services have been provided by NICE (142). Recent data from the UK highlighted the variation in practice, including the team members in the clinic, the age of transition and transfer, and who was responsible for care at different points in the process (143). Longer-term outcomes for patients who have a structured transition care appear to be improved, and education of paediatric patients to empower self-care is an important aspect of enabling IBD to be successfully treated within adult care (144).
Cancer data
One of the most feared complications of IBD is the risk of malignancy, although the absolute risk is extremely low. During childhood the risk is extraordinarily low, although there are cases of treatment-induced lymphoma, driven by thiopurines and biologics (73,145). Beyond 18 years, data would indicate that there is an increased risk of most types of cancer in patients with paediatric-onset disease, although the absolute risk remains extremely small (146). Data from numerous meta-analyses indicates that the most associated cancer type, colorectal carcinoma in ulcerative colitis, is only marginally increased in patients (2.4× compared with general population), with extensive disease and younger age at onset being risk factors (147,148). These are important considerations when discussing diagnoses with patients as the absolute risk of cancer in paediatric-onset disease remains extremely low.
Conclusions
The landscape of pIBD is changing, with increased utilisation of new therapies, advances diagnostics and improved understanding of disease pathogenesis. Despite this, evidence to suggest vast improvements in outcomes is lacking. Over the next 5–10 years ushering in an era of personalised therapy must the target for researchers and clinicians focused on pIBD.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-210/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-210/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-210/coif). JJA reports that he has received payment for being on the scientific advisory board of Orchard Therapeutics and is funded by an NIHR Advanced Fellowship. The other authors have no conflicts of interest to declare.
References
- 1.Kuenzig ME, Fung SG, Marderfeld L, et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022;162:1147-1159.e4. 10.1053/j.gastro.2021.12.282 [DOI] [PubMed] [Google Scholar]
- 2.Ashton JJ, Mossotto E, Ennis S, et al. Personalising medicine in inflammatory bowel disease-current and future perspectives. Transl Pediatr 2019;8:56-69. 10.21037/tp.2018.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nambu R, Warner N, Mulder DJ, et al. A Systematic Review of Monogenic Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 2022;20:e653-63. 10.1016/j.cgh.2021.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steenholdt C, Dige Ovesen P, Brynskov J, et al. Tofacitinib for Acute Severe Ulcerative Colitis: A Systematic Review. J Crohns Colitis 2023;17:1354-63. 10.1093/ecco-jcc/jjad036 [DOI] [PubMed] [Google Scholar]
- 5.Shah P, McDonald D. Vedolizumab: An Emerging Treatment Option for Pediatric Inflammatory Bowel Disease. J Pediatr Pharmacol Ther 2021;26:795-801. 10.5863/1551-6776-26.8.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyams JS, Davis Thomas S, Gotman N, et al. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study. Lancet 2019;393:1708-20. 10.1016/S0140-6736(18)32592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sýkora J, Pomahačová R, Kreslová M, et al. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol 2018;24:2741-63. 10.3748/wjg.v24.i25.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashton JJ, Barakat FM, Barnes C, et al. Incidence and Prevalence of Paediatric Inflammatory Bowel Disease Continues to Increase in the South of England. J Pediatr Gastroenterol Nutr 2022;75:e20-4. 10.1097/MPG.0000000000003511 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wei X, Sun Y, et al. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am J Physiol Gastrointest Liver Physiol 2019;317:G453-62. 10.1152/ajpgi.00103.2019 [DOI] [PubMed] [Google Scholar]
- 10.Sigall-Boneh R, Levine A, Lomer M, et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO]. J Crohns Colitis 2017;11:1407-19. [DOI] [PubMed] [Google Scholar]
- 11.Čipčić Paljetak H, Barešić A, Panek M, et al. Gut microbiota in mucosa and feces of newly diagnosed, treatment-naïve adult inflammatory bowel disease and irritable bowel syndrome patients. Gut Microbes 2022;14:2083419. 10.1080/19490976.2022.2083419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020;578:527-39. 10.1038/s41586-020-2025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashton JJ, Boukas K, Davies J, et al. Ileal Transcriptomic Analysis in Paediatric Crohn's Disease Reveals IL17- and NOD-signalling Expression Signatures in Treatment-naïve Patients and Identifies Epithelial Cells Driving Differentially Expressed Genes. J Crohns Colitis 2021;15:774-86. 10.1093/ecco-jcc/jjaa236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashton JJ, Boukas K, Stafford IS, et al. Deleterious Genetic Variation Across the NOD Signaling Pathway Is Associated With Reduced NFKB Signaling Transcription and Upregulation of Alternative Inflammatory Transcripts in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis 2022;28:912-22. 10.1093/ibd/izab318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 2014;15:382-92. 10.1016/j.chom.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moustafa A, Li W, Anderson EL, et al. Genetic risk, dysbiosis, and treatment stratification using host genome and gut microbiome in inflammatory bowel disease. Clin Transl Gastroenterol 2018;9:e132. 10.1038/ctg.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.G N , Zilbauer M. Epigenetics in IBD: a conceptual framework for disease pathogenesis. Frontline Gastroenterol 2022;13:e22-7. 10.1136/flgastro-2022-102120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floyd DN, Langham S, Séverac HC, et al. The economic and quality-of-life burden of Crohn's disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci 2015;60:299-312. 10.1007/s10620-014-3368-z [DOI] [PubMed] [Google Scholar]
- 19.Saha A, Tighe MP, Batra A. How to use faecal calprotectin in management of paediatric inflammatory bowel disease. Arch Dis Child Educ Pract Ed 2016;101:124-8. 10.1136/archdischild-2014-307941 [DOI] [PubMed] [Google Scholar]
- 20.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child 2003;88:995-1000. 10.1136/adc.88.11.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwell J, Saxena S, Jayasooriya N, et al. Prevalence and duration of gastrointestinal symptoms before diagnosis of Inflammatory Bowel Disease and predictors of timely specialist review: a population-based study. J Crohns Colitis 2020;jjaa146. 10.1093/ecco-jcc/jjaa146 [DOI] [PubMed] [Google Scholar]
- 22.Ashton JJ, Borca F, Mossotto E, et al. Analysis and Hierarchical Clustering of Blood Results Before Diagnosis in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis 2020;26:469-75. 10.1093/ibd/izy369 [DOI] [PubMed] [Google Scholar]
- 23.Oord T, Hornung N. Fecal calprotectin in healthy children. Scand J Clin Lab Invest 2014;74:254-8. 10.3109/00365513.2013.879732 [DOI] [PubMed] [Google Scholar]
- 24.Davidson F, Lock RJ. Paediatric reference ranges for faecal calprotectin: a UK study. Ann Clin Biochem 2017;54:214-8. 10.1177/0004563216639335 [DOI] [PubMed] [Google Scholar]
- 25.Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. Diagnostics guidance. 2013. Available online: https://www.nice.org.uk/guidance/dg11
- 26.Dilillo D, Zuccotti GV, Galli E, et al. Noninvasive testing in the management of children with suspected inflammatory bowel disease. Scand J Gastroenterol 2019;54:586-91. 10.1080/00365521.2019.1604799 [DOI] [PubMed] [Google Scholar]
- 27.Orfei M, Gasparetto M, Hensel KO, et al. Guidance on the interpretation of faecal calprotectin levels in children. PLoS One 2021;16:e0246091. 10.1371/journal.pone.0246091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjarnason I. The Use of Fecal Calprotectin in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2017;13:53-6. [PMC free article] [PubMed] [Google Scholar]
- 29.Brookes MJ, Whitehead S, Gaya DR, et al. Practical guidance on the use of faecal calprotectin. Frontline Gastroenterol 2018;9:87-91. 10.1136/flgastro-2016-100762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1-106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner D, Levine A, Escher JC, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr 2012;55:340-61. 10.1097/MPG.0b013e3182662233 [DOI] [PubMed] [Google Scholar]
- 32.Song JY, Lee YM, Choi YJ, et al. Fecal calprotectin level in healthy children aged less than 4 years in South Korea. J Clin Lab Anal 2017;31:e22113. 10.1002/jcla.22113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asgarshirazi M, Shariat M, Nayeri F, et al. Comparison of Fecal Calprotectin in Exclusively Breastfed and Formula or Mixed Fed Infants in the First Six Months of Life. Acta Med Iran 2017;55:53-8. [PubMed] [Google Scholar]
- 34.Roca M, Rodriguez Varela A, Donat E, et al. Fecal Calprotectin and Eosinophil-derived Neurotoxin in Healthy Children Between 0 and 12 Years. J Pediatr Gastroenterol Nutr 2017;65:394-8. 10.1097/MPG.0000000000001542 [DOI] [PubMed] [Google Scholar]
- 35.Roca M, Rodriguez Varela A, Carvajal E, et al. Fecal calprotectin in healthy children aged 4-16 years. Sci Rep 2020;10:20565. 10.1038/s41598-020-77625-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velasco Rodríguez-Belvís M, Viada Bris JF, Plata Fernández C, et al. Normal fecal calprotectin levels in healthy children are higher than in adults and decrease with age. Paediatr Child Health 2020;25:286-92. 10.1093/pch/pxz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014;58:795-806. 10.1097/MPG.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 38.Oliva S, Thomson M, de Ridder L, et al. Endoscopy in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto IBD Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:414-30. 10.1097/MPG.0000000000002092 [DOI] [PubMed] [Google Scholar]
- 39.Oliva S, Cohen SA, Di Nardo G, et al. Capsule endoscopy in pediatrics: a 10-years journey. World J Gastroenterol 2014;20:16603-8. 10.3748/wjg.v20.i44.16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalian M, Ozturk A, Oliva-Hemker M, et al. MR enterography findings of inflammatory bowel disease in pediatric patients. AJR Am J Roentgenol 2011;196:W810-6. 10.2214/AJR.10.5474 [DOI] [PubMed] [Google Scholar]
- 41.Tillack C, Seiderer J, Brand S, et al. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn's disease. Inflamm Bowel Dis 2008;14:1219-28. 10.1002/ibd.20466 [DOI] [PubMed] [Google Scholar]
- 42.Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's disease: a meta-analysis. Am J Gastroenterol 2010;105:1240-9. 10.1038/ajg.2009.713 [DOI] [PubMed] [Google Scholar]
- 43.Cohen SA, Oliva S. Capsule Endoscopy in Children. Front Pediatr 2021;9:664722. 10.3389/fped.2021.664722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atay O, Mahajan L, Kay M, et al. Risk of capsule endoscope retention in pediatric patients: a large single-center experience and review of the literature. J Pediatr Gastroenterol Nutr 2009;49:196-201. 10.1097/MPG.0b013e3181926b01 [DOI] [PubMed] [Google Scholar]
- 45.Bandorski D, Kurniawan N, Baltes P, et al. Contraindications for video capsule endoscopy. World J Gastroenterol 2016;22:9898-908. 10.3748/wjg.v22.i45.9898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570-83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 47.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179-207. 10.1016/j.crohns.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 48.Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ 2017;357:j2083. 10.1136/bmj.j2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miley D, Machado LB, Condo C, et al. Video Capsule Endoscopy and Ingestible Electronics: Emerging Trends in Sensors, Circuits, Materials, Telemetry, Optics, and Rapid Reading Software. Adv Devices Instrum 2021;2021:1-30. [Google Scholar]
- 50.Amil-Dias J, Kolacek S, Turner D, et al. Surgical Management of Crohn Disease in Children: Guidelines From the Paediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr 2017;64:818-35. 10.1097/MPG.0000000000001562 [DOI] [PubMed] [Google Scholar]
- 51.van Rheenen PF, Aloi M, Assa A, et al. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis 2020;jjaa161. 10.1093/ecco-jcc/jjaa161 [DOI] [PubMed] [Google Scholar]
- 52.Serrano-Moreno C, Brox-Torrecilla N, Arhip L, et al. Diets for inflammatory bowel disease: What do we know so far? Eur J Clin Nutr 2022;76:1222-33. 10.1038/s41430-021-01051-9 [DOI] [PubMed] [Google Scholar]
- 53.Hansen T, Duerksen DR. Enteral Nutrition in the Management of Pediatric and Adult Crohn's Disease. Nutrients 2018;10:537. 10.3390/nu10050537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miele E, Shamir R, Aloi M, et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;66:687-708. 10.1097/MPG.0000000000001896 [DOI] [PubMed] [Google Scholar]
- 55.Swaminath A, Feathers A, Ananthakrishnan AN, et al. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn's disease. Aliment Pharmacol Ther 2017;46:645-56. 10.1111/apt.14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cucinotta U, Romano C, Dipasquale V. Diet and Nutrition in Pediatric Inflammatory Bowel Diseases. Nutrients 2021;13:655. 10.3390/nu13020655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson T, Macdonald S, Hill SM, et al. Treatment of active Crohn's disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 2006;55:356-61. 10.1136/gut.2004.062554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.González-Torres L, Moreno-Álvarez A, Fernández-Lorenzo AE, et al. The Role of Partial Enteral Nutrition for Induction of Remission in Crohn's Disease: A Systematic Review of Controlled Trials. Nutrients 2022;14:5263. 10.3390/nu14245263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasson AN, Ananthakrishnan AN, Raman M. Diet in Treatment of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2021;19:425-35.e3. 10.1016/j.cgh.2019.11.054 [DOI] [PubMed] [Google Scholar]
- 60.Svolos V, Hansen R, Nichols B, et al. Treatment of Active Crohn's Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019;156:1354-67.e6. 10.1053/j.gastro.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 61.Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflamm Bowel Dis 2014;20:1353-60. 10.1097/MIB.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 62.Levine A, Wine E, Assa A, et al. Crohn's Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019;157:440-50.e8. 10.1053/j.gastro.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 63.Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:257-91. Erratum in: J Pediatr Gastroenterol Nutr 2020;71:794. 10.1097/MPG.0000000000002035 [DOI] [PubMed] [Google Scholar]
- 64.Caron B, Sandborn WJ, Panaccione R, et al. Efficacy of Pharmacological Agents for Ulcerative Proctitis: A Systematic Literature Review. J Crohns Colitis 2022;16:922-30. 10.1093/ecco-jcc/jjab218 [DOI] [PubMed] [Google Scholar]
- 65.Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf 2016;15:457-65. 10.1517/14740338.2016.1140743 [DOI] [PubMed] [Google Scholar]
- 66.Lim WC, Wang Y, MacDonald JK, et al. Aminosalicylates for induction of remission or response in Crohn's disease. Cochrane Database Syst Rev 2016;7:CD008870. 10.1002/14651858.CD008870.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernstein CN, Tenakoon A, Singh H, et al. Continued 5ASA use after initiation of anti-TNF or immunomodulator confers no benefit in IBD: a population-based study. Aliment Pharmacol Ther 2021;54:814-32. 10.1111/apt.16518 [DOI] [PubMed] [Google Scholar]
- 68.Chande N, Townsend CM, Parker CE, et al. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2016;10:CD000545. 10.1002/14651858.CD000545.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim SZ, Chua EW. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front Pharmacol 2018;9:1107. 10.3389/fphar.2018.01107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner D, Doveh E, Cohen A, et al. Efficacy of oral methotrexate in paediatric Crohn's disease: a multicentre propensity score study. Gut 2015;64:1898-904. 10.1136/gutjnl-2014-307964 [DOI] [PubMed] [Google Scholar]
- 71.Walker GJ, Harrison JW, Heap GA, et al. Association of Genetic Variants in NUDT15 With Thiopurine-Induced Myelosuppression in Patients With Inflammatory Bowel Disease. JAMA 2019;321:773-85. 10.1001/jama.2019.0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coelho T, Andreoletti G, Ashton JJ, et al. Genes implicated in thiopurine-induced toxicity: Comparing TPMT enzyme activity with clinical phenotype and exome data in a paediatric IBD cohort. Sci Rep 2016;6:34658. 10.1038/srep34658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon J, Ramaswami A, Beuttler M, et al. EBV Status and Thiopurine Use in Pediatric IBD. J Pediatr Gastroenterol Nutr 2016;62:711-4. 10.1097/MPG.0000000000001077 [DOI] [PubMed] [Google Scholar]
- 74.Warner B, Johnston E, Arenas-Hernandez M, et al. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol 2018;9:10-5. 10.1136/flgastro-2016-100738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn's Disease. Gastroenterology 2020;158:189-99. 10.1053/j.gastro.2019.09.041 [DOI] [PubMed] [Google Scholar]
- 76.Aardoom MA, Veereman G, de Ridder L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int J Mol Sci 2019;20:2529. 10.3390/ijms20102529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jongsma MME, Aardoom MA, Cozijnsen MA, et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn's disease: an open-label multicentre randomised controlled trial. Gut 2022;71:34-42. 10.1136/gutjnl-2020-322339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papamichael K, Dubinsky MC, Cheifetz AS. Therapeutic Drug Monitoring vs Standard Therapy During Infliximab Induction in Patients With Chronic Immune-Mediated Inflammatory Diseases. JAMA 2021;326:1067. 10.1001/jama.2021.11477 [DOI] [PubMed] [Google Scholar]
- 79.Boyapati RK, Ho GT, Satsangi J. Top-down in the Long Term in Crohn's Disease. J Crohns Colitis 2018;12:513-4. 10.1093/ecco-jcc/jjy027 [DOI] [PubMed] [Google Scholar]
- 80.Roda G, Jharap B, Neeraj N, et al. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol 2016;7:e135. 10.1038/ctg.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen NH, Solitano V, Vuyyuru SK, et al. Proactive Therapeutic Drug Monitoring Versus Conventional Management for Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2022;163:937-49.e2. 10.1053/j.gastro.2022.06.052 [DOI] [PubMed] [Google Scholar]
- 82.Papamichael K, Afif W, Drobne D, et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol Hepatol 2022;7:171-85. 10.1016/S2468-1253(21)00223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2017;376:1723-36. 10.1056/NEJMoa1606910 [DOI] [PubMed] [Google Scholar]
- 84.Girard C, Dirks M, Deslandres C. Tofacitinib to Treat Severe Acute Refractory Colitis in a Teenager: Case Report and Review of the Literature. JPGN Rep 2022;3:e241. 10.1097/PG9.0000000000000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh N, Rabizadeh S, Jossen J, et al. Multi-Center Experience of Vedolizumab Effectiveness in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis 2016;22:2121-6. 10.1097/MIB.0000000000000865 [DOI] [PubMed] [Google Scholar]
- 86.Dhaliwal J, McKay HE, Deslandres C, et al. One-year outcomes with ustekinumab therapy in infliximab-refractory paediatric ulcerative colitis: a multicentre prospective study. Aliment Pharmacol Ther 2021;53:1300-8. 10.1111/apt.16388 [DOI] [PubMed] [Google Scholar]
- 87.Wong ECL, Dulai PS, Marshall JK, et al. Comparative Efficacy of Infliximab vs Ustekinumab for Maintenance of Clinical Response in Biologic Naïve Crohn’s Disease. Inflamm Bowel Dis 2023;29:1015-23. 10.1093/ibd/izac168 [DOI] [PubMed] [Google Scholar]
- 88.Shim HH, Chan PW, Chuah SW, et al. A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases. JGH Open 2018;2:223-34. 10.1002/jgh3.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandborn WJ, Feagan BG, D'Haens G, et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2021;385:1280-91. 10.1056/NEJMoa2033617 [DOI] [PubMed] [Google Scholar]
- 90.Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022;399:2113-28. 10.1016/S0140-6736(22)00581-5 [DOI] [PubMed] [Google Scholar]
- 91.Mishra S, Jena A, Kakadiya R, et al. Positioning of tofacitinib in treatment of ulcerative colitis: a global perspective. Expert Rev Gastroenterol Hepatol 2022;16:737-52. 10.1080/17474124.2022.2106216 [DOI] [PubMed] [Google Scholar]
- 92.Collen LV. Rapid Clinical Remission With Upadacitinib in a Pediatric Patient With Refractory Crohn's Disease. Inflamm Bowel Dis 2023;29:1175-6. 10.1093/ibd/izad048 [DOI] [PubMed] [Google Scholar]
- 93.Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-based Consensus Guideline From the European Crohn's and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:292-310. 10.1097/MPG.0000000000002036 [DOI] [PubMed] [Google Scholar]
- 94.Torrente F, Meade S, Benchimol EI, et al. Thromboprophylaxis Use in Paediatric Inflammatory Bowel Disease: An International RAND Appropriateness Panel. J Crohns Colitis 2022;16:1609-16. 10.1093/ecco-jcc/jjac073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jena A, Mishra S, Sachan A, et al. Tofacitinib in Acute Severe Ulcerative Colitis: Case Series and a Systematic Review. Inflamm Bowel Dis 2021;27:e101-3. 10.1093/ibd/izab087 [DOI] [PubMed] [Google Scholar]
- 96.Constant BD, Baldassano R, Kirsch J, et al. Tofacitinib Salvage Therapy for Children Hospitalized for Corticosteroid- and Biologic-Refractory Ulcerative Colitis. J Pediatr Gastroenterol Nutr 2022;75:724-30. 10.1097/MPG.0000000000003616 [DOI] [PubMed] [Google Scholar]
- 97.Marsal J, Barreiro-de Acosta M, Blumenstein I, et al. Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front Med (Lausanne) 2022;9:897936. 10.3389/fmed.2022.897936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341-53. 10.1016/S2468-1253(19)30012-3 [DOI] [PubMed] [Google Scholar]
- 99.Khan S, Rupniewska E, Neighbors M, et al. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: A systematic review. J Clin Pharm Ther 2019;44:495-507. 10.1111/jcpt.12830 [DOI] [PubMed] [Google Scholar]
- 100.Guariso G, Gasparetto M. Treating children with inflammatory bowel disease: Current and new perspectives. World J Gastroenterol 2017;23:5469-85. 10.3748/wjg.v23.i30.5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sethi S, Dias S, Kumar A, et al. Meta-analysis: The efficacy of therapeutic drug monitoring of anti-TNF-therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2023;57:1362-74. 10.1111/apt.17313 [DOI] [PubMed] [Google Scholar]
- 102.Kansen HM, van Rheenen PF, Houwen RHJ, et al. Less Anti-infliximab Antibody Formation in Paediatric Crohn Patients on Concomitant Immunomodulators. J Pediatr Gastroenterol Nutr 2017;65:425-9. 10.1097/MPG.0000000000001551 [DOI] [PubMed] [Google Scholar]
- 103.Shmais M, Regueiro M, Hashash JG. Proactive versus Reactive Therapeutic Drug Monitoring: Why, When, and How? Inflamm Intest Dis 2022;7:50-8. 10.1159/000518755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bank S, Julsgaard M, Abed OK, et al. Polymorphisms in the NFkB, TNF-alpha, IL-1beta, and IL-18 pathways are associated with response to anti-TNF therapy in Danish patients with inflammatory bowel disease. Aliment Pharmacol Ther 2019;49:890-903. 10.1111/apt.15187 [DOI] [PubMed] [Google Scholar]
- 105.Bossuyt P, Pouillon L, Claeys S, et al. Ultra-proactive Therapeutic Drug Monitoring of Infliximab Based on Point of Care Testing in Inflammatory Bowel Disease: Results of a Pragmatic Trial. J Crohns Colitis 2022;16:199-206. 10.1093/ecco-jcc/jjab127 [DOI] [PubMed] [Google Scholar]
- 106.Ashton JJ, Cheng G, Stafford IS, et al. Prediction of Crohn’s Disease Stricturing Phenotype Using a NOD2-derived Genomic Biomarker. Inflamm Bowel Dis 2022;29:511-21. 10.1093/ibd/izac205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.NHS England. Improving outcomes through personalised medicine. Working at the cutting edge of science to improve patients’ lives. 2017. Available online: https://www.england.nhs.uk/wp-content/uploads/2016/09/improving-outcomes-personalised-medicine.pdf
- 108.Kammermeier J, Lamb CA, Jones KDJ, et al. Genomic diagnosis and care co-ordination for monogenic inflammatory bowel disease in children and adults: consensus guideline on behalf of the British Society of Gastroenterology and British Society of Paediatric Gastroenterology, Hepatology and Nutrition. Lancet Gastroenterol Hepatol 2023;8:271-86. 10.1016/S2468-1253(22)00337-5 [DOI] [PubMed] [Google Scholar]
- 109.Ashton JJ, Coelho T, Ennis S, et al. Presenting phenotype of paediatric inflammatory bowel disease in Wessex, Southern England 2010-2013. Acta Paediatr 2015;104:831-7. 10.1111/apa.13017 [DOI] [PubMed] [Google Scholar]
- 110.Griffiths AM, Nguyen P, Smith C, et al. Growth and clinical course of children with Crohn's disease. Gut 1993;34:939-43. 10.1136/gut.34.7.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashton JJ, Green Z, Young A, et al. Growth failure is rare in a contemporary cohort of paediatric inflammatory bowel disease patients. Acta Paediatr 2021;110:326-34. 10.1111/apa.15383 [DOI] [PubMed] [Google Scholar]
- 112.Mouratidou N, Malmborg P, Sachs MC, et al. Adult height in patients with childhood-onset inflammatory bowel disease: a nationwide population-based cohort study. Aliment Pharmacol Ther 2020;51:789-800. 10.1111/apt.15667 [DOI] [PubMed] [Google Scholar]
- 113.Church PC, Guan J, Walters TD, et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis 2014;20:1177-86. 10.1097/MIB.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 114.Gavin J, Ashton JJ, Heather N, et al. Nutritional support in paediatric Crohn's disease: outcome at 12 months. Acta Paediatr 2018;107:156-62. 10.1111/apa.14075 [DOI] [PubMed] [Google Scholar]
- 115.Dhaliwal J, Walters TD, Mack DR, et al. Phenotypic Variation in Paediatric Inflammatory Bowel Disease by Age: A Multicentre Prospective Inception Cohort Study of the Canadian Children IBD Network. J Crohns Colitis 2020;14:445-54. 10.1093/ecco-jcc/jjz106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Assa A, Rinawi F, Shamir R. The Long-Term Predictive Properties of the Paris Classification in Paediatric Inflammatory Bowel Disease Patients. J Crohns Colitis 2018;12:39-47. 10.1093/ecco-jcc/jjx125 [DOI] [PubMed] [Google Scholar]
- 117.Wintjens D, Bergey F, Saccenti E, et al. Disease Activity Patterns of Crohn's Disease in the First Ten Years After Diagnosis in the Population-based IBD South Limburg Cohort. J Crohns Colitis 2021;15:391-400. 10.1093/ecco-jcc/jjaa173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Irwin J, Ferguson E, Simms LA, et al. A rolling phenotype in Crohn's disease. PLoS One 2017;12:e0174954. 10.1371/journal.pone.0174954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeuring SF, van den Heuvel TR, Liu LY, et al. Improvements in the Long-Term Outcome of Crohn's Disease Over the Past Two Decades and the Relation to Changes in Medical Management: Results from the Population-Based IBDSL Cohort. Am J Gastroenterol 2017;112:325-36. 10.1038/ajg.2016.524 [DOI] [PubMed] [Google Scholar]
- 120.Ashton JJ, Borca F, Mossotto E, et al. Increased prevalence of anti-TNF therapy in paediatric inflammatory bowel disease is associated with a decline in surgical resections during childhood. Aliment Pharmacol Ther 2019;49:398-407. 10.1111/apt.15094 [DOI] [PubMed] [Google Scholar]
- 121.Bethell GS, Ashton JJ, Adams S, et al. The Influence of the Introduction of Biologic Agents on Surgical Intervention in Paediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 2022;75:308-12. 10.1097/MPG.0000000000003510 [DOI] [PubMed] [Google Scholar]
- 122.Ashton JJ, Beattie RM. Letter: anti-TNF therapy and intestinal resections in Crohn's disease-are we just delaying the inevitable?. Aliment Pharmacol Ther 2019;50:842-3. 10.1111/apt.15482 [DOI] [PubMed] [Google Scholar]
- 123.Kim HJ, Oh SH, Kim DY, et al. Clinical Characteristics and Long-Term Outcomes of Paediatric Crohn's Disease: A Single-Centre Experience. J Crohns Colitis 2017;11:157-64. 10.1093/ecco-jcc/jjw146 [DOI] [PubMed] [Google Scholar]
- 124.Papamichael K, Vande Casteele N, Gils A, et al. Long-term outcome of patients with Crohn's disease who discontinued infliximab therapy upon clinical remission. Clin Gastroenterol Hepatol 2015;13:1103-10. 10.1016/j.cgh.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 125.de Zoeten EF, Pasternak BA, Mattei P, et al. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr 2013;57:401-12. 10.1097/MPG.0b013e3182a025ee [DOI] [PubMed] [Google Scholar]
- 126.Turner D, Griffiths AM. Acute severe ulcerative colitis in children: a systematic review. Inflamm Bowel Dis 2011;17:440-9. 10.1002/ibd.21383 [DOI] [PubMed] [Google Scholar]
- 127.Ashton JJ, Versteegh HP, Batra A, et al. Colectomy in pediatric ulcerative colitis: A single center experience of indications, outcomes, and complications. J Pediatr Surg 2016;51:277-81. 10.1016/j.jpedsurg.2015.10.077 [DOI] [PubMed] [Google Scholar]
- 128.Hercun J, Willems P, Bilodeau M, et al. Long-Term Follow-Up into Adulthood of Pediatric-Onset Primary Sclerosing Cholangitis and Autoimmune Sclerosing Cholangitis. JPGN Rep 2022;3:e220. 10.1097/PG9.0000000000000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rinawi F, Assa A, Eliakim R, et al. Risk of Colectomy in Patients With Pediatric-onset Ulcerative Colitis. J Pediatr Gastroenterol Nutr 2017;65:410-5. 10.1097/MPG.0000000000001545 [DOI] [PubMed] [Google Scholar]
- 130.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet 2017;389:1710-8. 10.1016/S0140-6736(17)30317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Herman Y, Rinawi F, Rothschild B, et al. The Characteristics and Long-term Outcomes of Pediatric Crohn's Disease Patients with Perianal Disease. Inflamm Bowel Dis 2017;23:1659-65. 10.1097/MIB.0000000000001171 [DOI] [PubMed] [Google Scholar]
- 132.Akintimehin AO, O'Neill RS, Ring C, et al. Outcomes of a National Cohort of Children with Acute Severe Ulcerative Colitis. Front Pediatr 2018;6:48. 10.3389/fped.2018.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krauthammer A, Tzivinikos C, Assa A, et al. P214 Long-term outcomes of paediatric patients admitted for Acute Severe Colitis - a multicentre study from the paediatric IBD porto group of ESPGHAN. J Crohns Colitis 2018;12:abstr S208. [DOI] [PubMed]
- 134.Ihekweazu FD, Fofanova T, Palacios R, et al. Progression to colectomy in the era of biologics: A single center experience with pediatric ulcerative colitis. J Pediatr Surg 2020;55:1815-23. 10.1016/j.jpedsurg.2020.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hyams JS, Brimacombe M, Haberman Y, et al. Clinical and Host Biological Factors Predict Colectomy Risk in Children Newly Diagnosed With Ulcerative Colitis. Inflamm Bowel Dis 2022;28:151-60. 10.1093/ibd/izab061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aloi M, Bramuzzo M, Norsa L, et al. Disease Activity Patterns in the First 5 Years After Diagnosis in Children With Ulcerative Colitis: A Population-Based Study. J Crohns Colitis 2021;15:367-74. 10.1093/ecco-jcc/jjaa203 [DOI] [PubMed] [Google Scholar]
- 137.Ley D, Leroyer A, Dupont C, et al. New Therapeutic Strategies Have Changed the Natural History of Pediatric Crohn's Disease: A Two-Decade Population-Based Study. Clin Gastroenterol Hepatol 2022;20:2588-97.e1. 10.1016/j.cgh.2022.01.051 [DOI] [PubMed] [Google Scholar]
- 138.Kori M, Avidan M, Topf-Olivestone C. Long-Term Follow-up and Predictors of Complicated Disease Behavior in Pediatric Crohn's Disease Patients. J Pediatr Gastroenterol Nutr 2022;74:471-5. 10.1097/MPG.0000000000003374 [DOI] [PubMed] [Google Scholar]
- 139.Jang J, Lee SH, Jeong IS, et al. Clinical Characteristics and Long-term Outcomes of Pediatric Ulcerative Colitis: A Single-Center Experience in Korea. Gut Liver 2022;16:236-45. 10.5009/gnl20337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nagra A, McGinnity PM, Davis N, et al. Implementing transition: Ready Steady Go. Arch Dis Child Educ Pract Ed 2015;100:313-20. 10.1136/archdischild-2014-307423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeisler B, Hyams JS. Transition of management in adolescents with IBD. Nat Rev Gastroenterol Hepatol 2014;11:109-15. 10.1038/nrgastro.2013.254 [DOI] [PubMed] [Google Scholar]
- 142.Transition from children’s to adults’ services for young people using health or social care services. NICE guideline. 2016. Available online: https://www.nice.org.uk/guidance/ng43 [DOI] [PubMed]
- 143.Ashton JJ, Narula P, Kiparissi F, et al. Transition Services for Paediatric Inflammatory Bowel Disease: A Multicentre Study of Practice in the United Kingdom. J Pediatr Gastroenterol Nutr 2021;73:251-8. 10.1097/MPG.0000000000003148 [DOI] [PubMed] [Google Scholar]
- 144.Bennett AL, Moore D, Bampton PA, et al. Outcomes and patients' perspectives of transition from paediatric to adult care in inflammatory bowel disease. World J Gastroenterol 2016;22:2611-20. 10.3748/wjg.v22.i8.2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2011;9:36-41.e1. 10.1016/j.cgh.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 146.Olén O, Askling J, Sachs MC, et al. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ 2017;358:j3951. 10.1136/bmj.j3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jess T, Simonsen J, Jørgensen KT, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143:375-81.e1; quiz e13-4. 10.1053/j.gastro.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 148.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012;10:639-45. 10.1016/j.cgh.2012.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as