Key Points
-
•
Germ line genetic predisposition to myeloid neoplasms significantly contributes to cytopenias and hypoplastic BM in adulthood.
-
•
Pathogenic/likely pathogenic germ line variants are associated with a higher risk of severe cytopenias and advanced myeloid neoplasms.
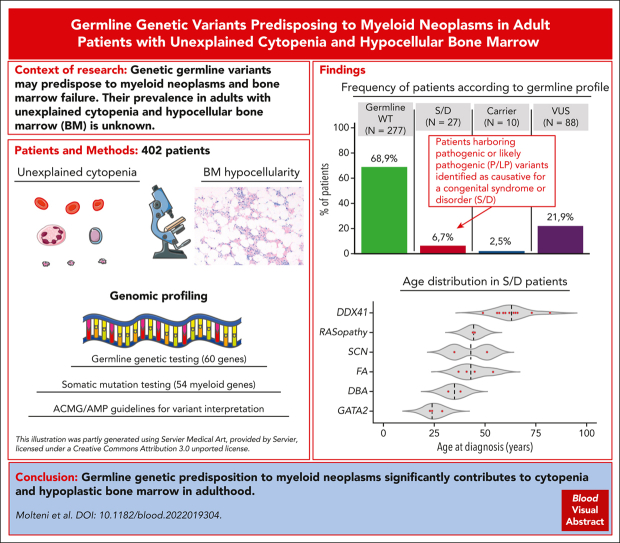
Visual Abstract
Abstract
Systematic studies of germ line genetic predisposition to myeloid neoplasms in adult patients are still limited. In this work, we performed germ line and somatic targeted sequencing in a cohort of adult patients with hypoplastic bone marrow (BM) to study germ line predisposition variants and their clinical correlates. The study population included 402 consecutive adult patients investigated for unexplained cytopenia and reduced age-adjusted BM cellularity. Germ line mutation analysis was performed using a panel of 60 genes, and variants were interpreted per the American College of Medical Genetics and Genomics/Association for Molecular Pathology guidelines; somatic mutation analysis was performed using a panel of 54 genes. Of the 402 patients, 27 (6.7%) carried germ line variants that caused a predisposition syndrome/disorder. The most frequent disorders were DDX41-associated predisposition, Fanconi anemia, GATA2-deficiency syndrome, severe congenital neutropenia, RASopathy, and Diamond-Blackfan anemia. Eighteen of 27 patients (67%) with causative germ line genotype were diagnosed with myeloid neoplasm, and the remaining with cytopenia of undetermined significance. Patients with a predisposition syndrome/disorder were younger than the remaining patients and had a higher risk of severe or multiple cytopenias and advanced myeloid malignancy. In patients with myeloid neoplasm, causative germ line mutations were associated with increased risk of progression into acute myeloid leukemia. Family or personal history of cancer did not show significant association with a predisposition syndrome/disorder. The findings of this study unveil the spectrum, clinical expressivity, and prevalence of germ line predisposition mutations in an unselected cohort of adult patients with cytopenia and hypoplastic BM.
In this month’s CME article, Molteni et al assess the contribution of germ line genetic disposition to cytopenias and hypocellular bone marrow in 402 adult patients. Germ line and somatic mutation analyses of a panel of 60 and 54 genes respectively revealed that 6.7% of these individuals carry germ line variants known to cause a predisposition syndrome; of these, 67% had a myeloid neoplasm, and the remainder had clonal cytopenia of undetermined significance. The study elucidates a spectrum of mutations that varies with age and predisposes to myeloid neoplasm.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 678.
Disclosures
CME questions author Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
-
1.
Assess the prevalence and spectrum of germ line predisposition to myeloid neoplasms and other genetic variants in adults with age-adjusted hypocellular bone marrow, based on a large cohort study using germ line and somatic targeted sequencing
-
2.
Determine the clinical correlates of predisposition syndromes/disorders and diagnostic implications of germ line mutations in adults with suspected myeloid neoplasm, based on a large cohort study using germ line and somatic targeted sequencing
-
3.
Evaluate the clinical implications of prevalence and phenotypic expressivity of germ line variants predisposing to myeloid neoplasms among adults with cytopenia and hypoplastic bone marrow, based on a large cohort study using germ line and somatic targeted sequencing
Release date: August 17, 2023; Expiration date: August 17, 2024
Introduction
Genetic germ line variants are increasingly recognized as predisposing factors to myeloid neoplasms and aplastic anemia (AA), and a category of myeloid neoplasms with germ line predisposition was included in the 2017 World Health Organization classification of hematopoietic tumors that comprises cases of myelodysplastic syndromes (MDSs), myelodysplastic/myeloproliferative neoplasms, and acute myeloid leukemia (AML) that occur with the background of a germ line mutation.1,2 According to this classification and recent proposals for revision, the underlying genetic defects or syndromes are classified based on the absence or presence of a preexisting clinical phenotype, reflecting variable penetrance and clinical expressivity of these conditions.1,3,4
The available studies on the frequency of germ line predisposition in patients with myeloid malignancies or bone marrow failure (BMF) mainly focused on cohorts of young adults who were selected based on clinical suspicion or family history.5,6 Although germ line testing is increasingly performed in the work-up of patients with suspected myeloid neoplasm and expert guidance has been offered,2,7, 8, 9, 10 systematic studies of the frequency of germ line predisposition in adult patients with MDS or AA are limited, and reliable clinical indicators driving appropriate genetic testing are lacking.11, 12, 13, 14 Although the age at diagnosis is an important criterion to identify individuals at risk,15 recent data consistently indicate that selected conditions, such as DDX41-associated susceptibility to myeloid neoplasms, may result in phenotypic expression in older individuals.16, 17, 18, 19, 20, 21
A hypoplastic bone marrow is a common feature of adult patients with late onset, inherited BMF syndromes.15 Bone marrow hypocellularity also characterizes acquired hematologic disorders, including AA and hypoplastic MDS, a condition with variable clinical features and outcome,22 which has recently been recognized as a distinct disease subtype.4 Bone marrow hypocellularity may lead to difficulties in the differential diagnosis, and, accordingly, it has also been reported in cytopenias of undetermined significance.22
In this work, we performed germ line and somatic targeted sequencing in a large, well-annotated cohort of adult patients with cytopenia and hypoplastic bone marrow to define prevalence and phenotypic expressivity of germ line variants predisposing to myeloid neoplasms.
Patients and methods
Patient characteristics and clinical procedures
This study included 402 consecutive adult patients investigated at the IRCCS Policlinico San Matteo Foundation and the University of Pavia, Pavia, Italy, based on the following criteria: (1) unexplained cytopenia defined based on standard hematologic values and (2) reduced age-adjusted bone marrow cellularity. Diagnostic procedures aimed at distinguishing myeloid neoplasms from reactive causes of cytopenia or other hematologic disorders were in accordance with recent recommendations.1,3,4,23, 24, 25 Age-adjusted bone marrow cellularity was assessed using a bone marrow trephine biopsy, per the current consensus-based recommendation.26 Idiopathic cytopenia of undetermined significance (ICUS) was defined as previously reported,27,28 whereas clonal cytopenia of undetermined significance (CCUS) was defined per recent recommendations.3,4,27 Peripheral blood and bone marrow specimens were analyzed by 2 independent hematopathologists who were blinded to clinical data; discordant results were resolved by a joint review of the specimens. Clinical and demographic features of the patients included in the study are reported in Table 1. A portion of patients belongs to a cohort that was previously reported in a study on the integration of cytohistological and somatic mutation profiles.22
Table 1.
Clinical and hematologic features of individuals included in the study
| Variable | |
|---|---|
| No. of patients | 402 |
| Age, y (median, range) | 56 (18-86) |
| Sex (female/male) | 203/199 |
| Hemoglobin, g/dL (median, range) | 11.8 (3.9-17.4) |
| MCV, fL (median, range) | 95 (60-124) |
| WBC, ×109/L (median, range) | 3.5 (0.5-28.51) |
| ANC, ×109/L (median, range) | 1.63 (0.09-9.77) |
| PLT, ×109/L (median, range) | 129 (1-666) |
| Diagnosis based on the standard work-up | |
| Idiopathic cytopenia of undetermined significance | 162 (40%) |
| Clonal cytopenia of undetermined significance | 26 (6%) |
| Myeloid neoplasm | 173 (43%) |
| Acute myeloid leukemia | 11 (6%) |
| MDS | 148 (86%) |
| MDS single-lineage dysplasia | 19 (13%) |
| MDS multilineage dysplasia | 60 (41%) |
| MDS with ring sideroblasts | 8 (5%) |
| MDS excess blasts | 51 (34%) |
| MDS del(5q) | 8 (6%) |
| MDS, unclassified | 2 (1%) |
| Myelodysplastic/myeloproliferative neoplasm | 14 (8%) |
| AA | 41 (10%) |
| Nonsevere | 27 (66%) |
| Severe | 14 (34%) |
| Bone marrow cellularity, % (median, range) | 25 (5-50) |
| ≤25% | 206 (51%) |
| Reduced for age | 196 (49%) |
ANC, absolute neutrophil count; MCV, mean corpuscular volume; MDS del(5q), myelodysplastic syndrome with isolated deletion of chromosome 5q; PLT, platelet count; WBC, white blood cell count.
This study was approved by the ethics committee of the IRCCS Policlinico San Matteo Foundation, Pavia, Italy. The procedures followed were in accordance with the Declaration of Helsinki of 1975, as revised in 2000, and samples were obtained after written informed consent.
Mutation analysis
Germ line mutation analysis was performed on 60 genes selected based on prior implication in predisposition to myeloid neoplasm (Supplemental Table 1, available on the Blood website) using a capture-based approach and the DNA Prep with Enrichment technology (Illumina, San Diego, CA). Peripheral blood granulocytes were analyzed as a tumor DNA source. Purified CD3+ T-lymphocytes and buccal cells were used as a germ line control tissue; the germ line origin of SAMD9/SAMD9L variants was confirmed in genomic DNA samples from buccal cells.29 Variants were analyzed using the Expert Variant Interpreter (eVai) (enGenome, Pavia, Italy) and manually curated before classification per the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) guidelines, and Clinical Genome Resource disease-associated gene specifications.30,31 Somatic mutation analysis was performed by next-generation sequencing of a core panel of 54 genes (supplemental Table 2), as previously described.32,33 Details of library preparation, sequencing, and variant analysis are provided in the supplemental Material.
Statistical analysis
Statistical analyses were performed using Stata SE 16.1 (StataCorp LP, College Station, TX; http://www.stata.com) and R 3.6.2 (https://www.r-project.org) software. Details of the statistical analysis are reported in the supplemental Material.
Results
Prevalence and spectrum of germ line predisposition to myeloid neoplasms in adults with age-adjusted hypocellular bone marrow
We studied the germ line predisposition to myeloid neoplasms in a cohort of 402 adult patients with cytopenia and hypocellular bone marrow, as defined by a reduced age-adjusted bone marrow cellularity (n = 196) and/or bone marrow cellularity ≤25% (n = 206), including 173 myeloid neoplasms (43%), and 229 ICUS/CCUS or nonmalignant BMF (57%).
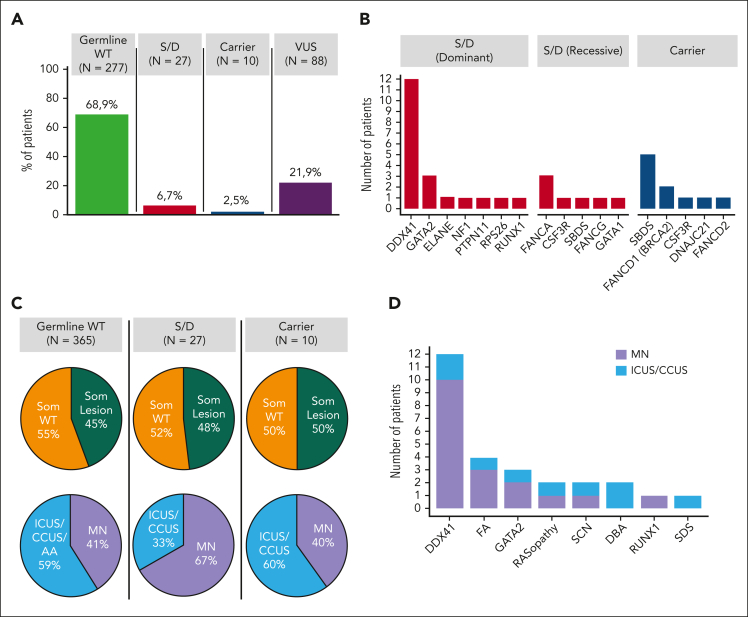
Of the 402 patients, 27 (6.7%) patients had germ line mutations classified as pathogenic/likely pathogenic (P/LP) per ACMG/AMP criteria resulting in a genotype consistent with a diagnosis of a congenital syndrome or disorder (Figure 1A; Table 2; supplemental Table 3). In detail, 20 of these patients harbored heterozygous mutations in genes that cause autosomal dominant disorders (ie, DDX41, GATA2, ELANE, NF1, PTPN11, RPS26, and RUNX1), whereas 6 had homozygous/compound heterozygous mutations in genes that cause autosomal recessive syndromes (ie, FANCA, CSF3R, SBDS, and FANCG). Finally, a male patient presented a hemizygous mutation in the 5′ untranslated region of the GATA1 gene, which causes an X-linked recessive condition (Figure 1B).
Figure 1.
Germ line mutation landscape, prevalence of somatic lesions, and myeloid malignancy diseases in the cohort included in the study. (A) Frequency of patients without germ line mutations in any of the 60 genes screened (green), patients harboring P/LP mutations identified as causative for a congenital syndrome or disorder (S/D) (red), patients carrying a unique heterozygous mutation in genes associated with autosomal recessive disorders (blue), or patients harboring VUS (purple). (B) Distribution of patients with S/D sustained by the heterozygous mutation in genes associated with dominant inheritance and of patients with S/D associated with homozygous/compound heterozygous mutations in genes associated with recessive inheritance (red bars) as well as of carriers of a unique heterozygous mutation in genes associated with autosomal recessive disorders (blue bars). (C) Pie charts depicting the prevalence of somatic lesions (mutations and/or cytogenetic abnormalities) (green, presence of somatic lesion[s]; orange, absence of somatic lesion[s]) (top), and the prevalence of myeloid neoplasm (MN) (lavender) or nonmalignant conditions (ICUS, CCUS, or AA) (light blue) (bottom), in patients with no germ line mutations (wild-type; WT), patients with S/D, and carriers of a unique heterozygous mutation in genes associated with autosomal recessive disorders. All patients without P/LP variants were included in the germ line WT group. (D) Distribution of the standard hematologic diagnosis (MN [lavender], and nonmalignant conditions [light blue]), in the 27 patients with S/D, based on the underlying syndromes/disorders. DBA, Diamond-Blackfan anemia; FA, Fanconi anemia; SCN, severe congenital neutropenia; SDS, Shwachman-Diamond syndrome.
Table 2.
Characteristics of patients with germ line variants classified as P/LP, per the ACMG/AMP criteria, and causative for a congenital syndrome/disorder
| Patient ID | Age/sex | Germ line gene mutation |
VAF myeloid | VAF Germ line | Congenital S/D | Somatic mutation |
Cytogenetics (% abnormal metaphases) | Hb g/dl | ANC, ×109/L | Plt, ×109/L | Diagnosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutated gene | VAF | |||||||||||
| PV1185 | 51/F |
CSF3R c.1474+1G>C CSF3R p.(W566∗) |
0.40 0.40 |
0.48 0.45 |
SCN | WT | 46XX | 13.2 | 0.5 | 275 | ICUS | |
| PV1395 | 82/M | DDX41 p.(M1?) | 0.46 | 0.48 | DDX41-associated predisposition | DDX41 | 0.11 | 45X;-Y (50) | 13 | 0.9 | 193 | MDS-MLD |
| PV1583 | 56/F | DDX41 p.(M1?) | 0.48 | 0.50 | DDX41-associated predisposition | SF3B1 | 0.02 | 46XX | 10.4 | 0.4 | 90 | MDS-EB-2 |
| PV1336 | 59/M | DDX41 p.(Q48∗) | 0.48 | 0.48 | DDX41-associated predisposition | WT | 46XY | 12.2 | 2.9 | 165 | MDS-EB-2 | |
| PV1475 | 66/M | DDX41 p.(K102fs) | 0.45 | 0.48 | DDX41-associated predisposition | WT | failed | NA | MDS-EB-2 | |||
| PV10015 | 62/M | DDX41 p.(K102fs) | 0.48 | 0.45 | DDX41-associated predisposition | WT | 46XY; del(7) add(4) (18) | 8.3 | 1.47 | 24 | AML | |
| PV1178 | 57/M | DDX41 p.(I207T) | 0.49 | 0.40 | DDX41-associated predisposition | DDX41 | 0.01 | 46XY | 10.1 | 0.5 | 47 | MDS-EB-2 |
| PV1581 | 64/M | DDX41 p.(I207T) | 0.46 | 0.48 | DDX41-associated predisposition | WT | 46XY | 11.4 | 0.8 | 48 | MDS-EB-2 | |
| PV693 | 49/M | DDX41 p.(G218D) | 0.49 | 0.50 | DDX41-associated predisposition |
ASXL1 STAG2 |
0.29 0.35 |
46XY | 14.4 | 1.76 | 80 | MDS-EB-2 |
| PV1591 | 66/M | DDX41 p.(G218D) | 0.48 | 0.49 | DDX41-associated predisposition | WT | 46XY | 11.8 | 1.67 | 181 | MDS-EB-2 | |
| PV2607 | 65/M | DDX41 p.(P258L) | 0.49 | 0.46 | DDX41-associated predisposition | WT | 46XY | 12.5 | 2.44 | 60 | ICUS | |
| PV583 | 73/M | DDX41 p.(Y259H) | 0.46 | 0.48 | DDX41-associated predisposition | DDX41 | 0.11 | 46XY | 11.6 | 1.5 | 101 | MDS-MLD |
| PV554 | 60/F | DDX41 p.(R267W) | 0.44 | 0.50 | DDX41-associated predisposition |
ASXL1 ATRX BCOR |
0.38 0.14 0.12 |
46XX | 11.1 | 1.65 | 40 | CCUS |
| PV1546 | 35/M | ELANE p.(R220Q) | 0.50 | 0.52 | SCN | WT | 46XY | 14 | 0.8 | 141 | MDS-MLD | |
| PV2613 | 54/F |
FANCA c.79+1G>C FANCA p.(P1324L) |
0.50 0.49 |
0.50 0.44 |
FA | WT | 46XX; trp(q21;q32) add(19)(p13) (100) | 10.6 | 0.8 | 33 | MDS-MLD | |
| PV2443 | 37/F | FANCA p.(V372fs)∗ | 0.99 | 0.97 | FA | WT | complex karyotype (100) | 12.5 | 0.7 | 106 | MDS-MLD | |
| PV1047 | 45/M | FANCA p.(N1140fs) | 1.00 | 0.98 | FA |
ASXL1 EZH2 STAG2 TET2 |
0.45 0.94 0.50 0.22 |
46XY/47XY; del(5q) del(17p) +mar (80) | 12 | 2.3 | 145 | MDS-EB-1 |
| PV10061 | 41/F | FANCG p.(E395fs) | 1.00 | 0.98 | FA | WT | 46XX | 10.6 | 1.4 | 110 | ICUS | |
| PV2292 | 32/M | GATA1 c.-19-2A>G∗ | 0.97 | 0.98 | DBA | WT | 46XY | 11.6 | 0.5 | 108 | ICUS | |
| PV2663 | 29/F | GATA2 p.(R330∗) | 0.50 | 0.46 | GATA2-deficiency syndrome | WT | 46XX | 9.5 | 1.2 | 148 | ICUS | |
| PV2274 | 23/F | GATA2 c.1018-1G>A | 0.56 | 0.54 | GATA2-deficiency syndrome | NPM1 | NA | 46XX | 10.7 | 31 | 215 | AML |
| PV1264 | 24/F | GATA2 p.(R398W) | 0.52 | 0.42 | GATA2-deficiency syndrome | STAG2 | 0.04 | 46XX | 8.1 | 0.8 | 58 | MDS-MLD |
| PV2661 | 45/M | NF1 c.3113+2T>A | 0.47 | 0.44 | NF |
RAF1 TET2 |
0.25 0.22 |
45X;-Y (60) | 8.4 | 2.2 | 184 | CMML-0 |
| PV2662 | 44/F | PTPN11 p.(T468M) | 0.51 | 0.51 | NS | WT | 46XX | 8.2 | 1.5 | 19 | ICUS | |
| PV2664 | 38/M | RPS26 p.(M1?) | 0.51 | 0.53 | DBA | WT | 46XY | 12.7 | 0.25 | 152 | ICUS | |
| PV1774 | 52/F | RUNX1 p.(L56fs)∗ | 0.50 | NA | RUNX1-related FPD | WT | 46XX | 5.9 | 2.0 | 111 | MDS/MPN-U | |
| PV2117 | 24/F |
SBDS p.(K62∗)∗ SBDS c.258+2T>C∗ |
0.48 0.54 |
0.40 0.52 |
SDS | WT | 46XX | 10.4 | 0.4 | 90 | ICUS | |
ANC, absolute neutrophil count; CMML, chronic myelomonocytic leukemia; DBA, Diamond-Blackfan anemia; F, female; FA, Fanconi anemia; FPD, familial platelet disorder; Hb, hemoglobin concentration; M, male; MDS-EB, MDS with excess blasts; MDS-MLD, MDS with multilineage dysplasia; MDS/MPN-U, MDS/myeloproliferative neoplasm unclassifiable; NA, not available; NF, neurofibromatosis; NS, Noonan syndrome; Plt, platelet count; SCN, severe congenital neutropenia; S/D, syndrome/disorder; SDS, Shwachman-Diamond syndrome; VAF, variant allele frequency; WT, wild-type.
FANCA p.(V372fs) variant was detected in an unaffected male (son), with VAF 0.43. GATA1 c.-19-2A>G variant was detected in an affected sibling, with VAF 0.50. RUNX1 p.(L56fs) variant was detected in an affected sibling, with VAF 0.54. SBDS p.(K62∗) and c.258+2T>C variants were detected in an affected sibling, with VAFs 0.48 and 0.40, respectively.
Congenital syndromes or disorders included DDX41-associated predisposition (n = 12 patients), followed by Fanconi anemia (FANCA and FANCG) (n = 4), GATA2-deficiency syndrome (n = 3), RASopathy (NF1 and PTPN11) (n = 2), severe congenital neutropenia (CSF3R and ELANE) (n = 2), Diamond-Blackfan anemia (GATA1 and RPS26) (n = 2), and RUNX1-related familial platelet disorder and Shwachman-Diamond syndrome (SBDS) (n = 1) (supplemental Results).
In addition, a unique heterozygous P/LP mutation in genes associated with autosomal recessive disorders was identified in 10 out of 402 (2.5%) cases (Figure 1A-B; Table 3).
Table 3.
Characteristics of patients carrying P/LP heterozygous germ line variants in genes involved in autosomal recessive disorders
| Patient ID | Age/sex | Germ line gene mutation |
VAF myeloid | VAF germ line | Congenital S/D | Somatic mutation |
Cytogenetics (% abnormal metaphases) | Hb g/dl | ANC, ×109/L | Plt, ×109/L | Diagnosis | Extrahematologic phenotype | Family history of cancer hem/solid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutated gene | VAF | |||||||||||||
| PV1410 | 70/M | CSF3R∗ p.(P146fs) | 0.43 | 0.46 | SCN |
JAK2 U2AF1 ZRSR2 |
0.10 0.12 0.15 |
Failed | 11.6 | 1.10 | 290 | MDS/MPN | + | −/− |
| PV1515 | 45/F | FANCD1 (BRCA2)∗ p.(Y2624C) | 0.44 | 0.48 | FA/HBOC | WT | Failed | 13.8 | 1.35 | 129 | ICUS | − | −/− | |
| PV1949 | 25/M | FANCD1 (BRCA2)∗ p.(Y3226fs) | 0.46 | 0.52 | FA/HBOC | WT | 46XY | 15.6 | 2.7 | 131 | ICUS | + | −/− | |
| PV2480 | 25/M | DNAJC21 p.(Ter577fs) | 0.53 | 0.50 | SDS | WT | 46XY | 13.2 | 0.57 | 151 | ICUS | + | −/− | |
| PV30044 | 50/M | FANCD2 p.(P679fs) | 0.48 | 0.45 | FA | DNMT3A | 0.10 | 46XY | 6 | 1.12 | 44 | AML | − | +/− |
| PV1174 | 38/F | SBDS c.258+2T>C† | 0.40 | 0.48 | SDS | WT | 46XX | 14.6 | 1.8 | 144 | ICUS | − | −/- | |
| PV1338 | 49/M | SBDS c.258+2T>C† | 0.40 | 0.48 | SDS |
ASXL1 NRAS WT1 WT1 |
0.43 0.04 0.36 0.03 |
46XY | 10.6 | 0.81 | 66 | AML | + | −/- |
| PV2281 | 70/F | SBDS c.258+2T>C† | 0.41 | 0.40 | SDS | TET2 | 0.38 | 46XX; del(5q) (60) | 11.6 | 1.24 | 173 | MDS del(5q) | + | −/− |
| PV2346 | 47/M | SBDS c.258+2T>C† | 0.41 | 0.41 | SDS |
PHF6 SRSF2 |
0.93 0.47 |
46XY | 12.5 | 6.4 | 35 | CCUS | − | −/− |
| PV2360 | 47/M | SBDS c.258+2T>C† | 0.40 | 0.40 | SDS | WT | 46XY | 12.8 | 2.5 | 209 | ICUS | − | −/+ | |
ANC, absolute neutrophil count; F, female; FA/HBOC, Fanconi anemia/hereditary breast and ovarian cancer syndrome; Hb, hemoglobin concentration; Hem, hematopoietic; M, male; MDS/MPN, MDS/myeloproliferative neoplasm; MDS del(5q), myelodysplastic syndrome with deletion of chromosome 5q; Plt, platelet count; SCN, severe congenital neutropenia; S/D, syndrome/disorder; SDS, Shwachman-Diamond syndrome; WT, wild-type; VAF, variant allele frequency.
Heterozygous CSF3R and FANCD1 (BRCA2) variants were described, associated with predisposition to hematologic malignancies.34, 35, 36
Manual revision of the BAM files on the SBDS gene and its pseudogene SBDSP1 confirmed the absence of the variant p.(K62∗) in patients heterozygous for c.258+2T>C.
Finally, 111 germ line variants of unknown significance (VUS) were identified in 38 genes in 88 out of 402 (21.9%) patients (Figure 1A; supplemental Figure 1; supplemental Table 4).
Penetrance of predisposition syndromes/disorders for hematologic phenotype or myeloid neoplasm and somatic clonal evolution
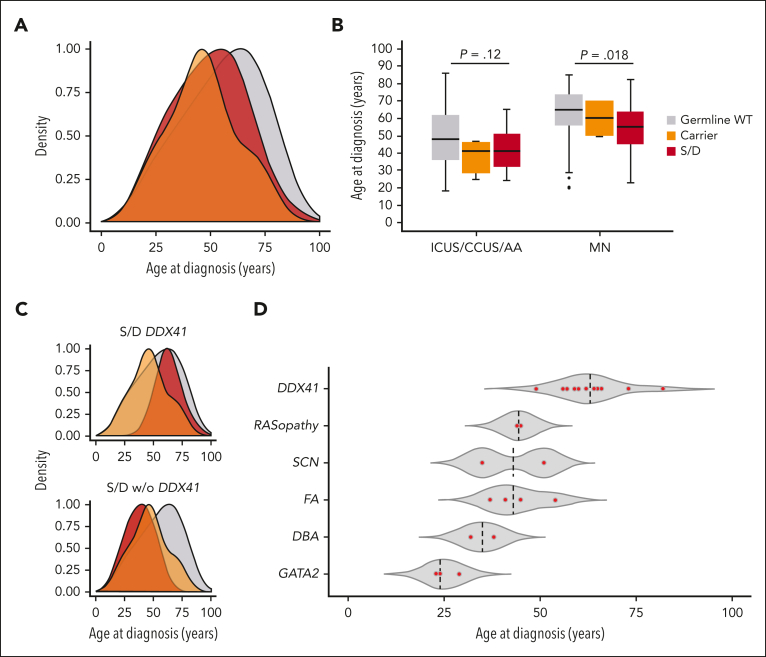
Of 27 patients (67%) with genotype causative for a congenital syndrome/disorder, 18 patients received a diagnosis of myeloid neoplasm, whereas 9 received a diagnosis of ICUS/CCUS (Figure 1C-D; Table 2), 2 of whom subsequently progressed to myeloid neoplasm. The median age at the time of diagnosis was 51 years (range, 23-82 years) (Figure 2A), with a male preponderance (15 of 27 patients). Overall, 9 patients (33%) had a family history of hematologic or extrahematologic malignancy, whereas 7 showed extrahematologic phenotype fostering the suspicion of a germ line disorder with multiorgan involvement (Table 4).
Figure 2.
Age distribution at the time of standard hematologic diagnosis in patients with or without germ line mutation. (A) Density curves representing the age distribution at the time of standard diagnosis in patients who are germ line WT in the analyzed genes (gray), patients harboring P/LP mutations identified as causative of a congenital syndrome or disorder (S/D) (red), or carriers of a unique heterozygous mutation in genes associated with autosomal recessive disorders (orange). (B) Box plots showing the median age (horizontal line) and age distribution at the time of diagnosis of MN or nonneoplastic conditions (ICUS/CCUS/AA), in patients who are germ line WT (gray), S/D (red), and carriers (orange). Patients who are germ line mutated were significantly younger at the time of diagnosis of MN than those who are germ line WT (P = .018). (C) Age distribution at the time of diagnosis in patients who are germ line WT (gray), carriers of a unique heterozygous mutation in genes associated with autosomal recessive disorders (orange), and patients with DDX41-associated predisposition (red) (top), or with S/D other than DDX41-associated predisposition (red) (bottom). (D) Age distribution based on the germ line S/D (red dots); median age is indicated with a dashed line.
Table 4.
Clinical phenotype and family history of patients with P/LP germ line, per ACMG/AMP criteria, causative of a congenital syndrome/disorder
| Patient ID | Age/sex | Germ line mutated gene |
Congenital syndrome/disorder | Diagnosis | Extrahematologic phenotype | Family history |
|
|---|---|---|---|---|---|---|---|
| Hematologic/solid cancer | Other | ||||||
| PV1185 | 51/F |
CSF3R CSF3R |
SCN | ICUS | Connective tissue disease treated with methotrexate | — | — |
| PV1395 | 82/M | DDX41 | DDX41-associated predisposition | MDS-MLD | Hyperthyroidism treated with radiometabolic therapy | — | — |
| PV1583 | 56/F | DDX41 | DDX41-associated predisposition | MDS-EB-2 | — | — | — |
| PV1336 | 59/M | DDX41 | DDX41-associated predisposition | MDS-EB-2 | — | — | — |
| PV1475 | 66/M | DDX41 | DDX41-associated predisposition | MDS-EB-2 | — | — | — |
| PV10015 | 62/M | DDX41 | DDX41-associated predisposition | AML | Colon cancer treated with chemotherapy | Mother with colon and uterine cancers | — |
| PV1178 | 57/M | DDX41 | DDX41-associated predisposition | MDS-EB-2 | — | — | — |
| PV1581 | 64/M | DDX41 | DDX41-associated predisposition | MDS-EB-2 | — | — | — |
| PV693 | 49/M | DDX41 | DDX41-associated predisposition | MDS-EB-2 | — | — | — |
| PV1591 | 66/M | DDX41 | DDX41-associated predisposition | MDS-EB-2 | Cardiomyopathy | — | — |
| PV2607 | 65/M | DDX41 | DDX41-associated predisposition | ICUS | Chronic gastritis HP+; APCA-positive | — | — |
| PV583 | 73/M | DDX41 | DDX41-associated predisposition | MDS-MLD | — | — | — |
| PV554 | 60/M | DDX41 | DDX41-associated predisposition | CCUS | — | — | — |
| PV1546 | 35/M | ELANE | SCN | MDS-MLD | Mild splenomegaly | Father with MDS died after bone marrow transplant | Brother with isolated neutropenia |
| PV2613 | 54/F |
FANCA FANCA |
FA | MDS-MLD | Short stature, triangular facies, small head café au lait spots, hypertrichosis, learning disabilities; spinocellular cell and genital carcinoma | Brother with BMF died at age 10 | Father with low platelet counts died of intracranial bleeding |
| PV2443 | 37/F | FANCA∗ | FA | MDS-MLD | Short stature; intrauterine fetal death (seventh month); hepatic steatosis | — | — |
| PV1047 | 45/M | FANCA | FA | MDS-EB-1 | — | — | — |
| PV10061 | 41/F | FANCG | FA | ICUS | Short stature intellectual disability, congenital right hearing loss, horseshoe kidney; polyabortivity; squamous cell carcinoma | One brother with ALL and 1 brother with esophagus cancer died at age 33; mother with breast cancer died at age 56 | — |
| PV2292 | 32/M | GATA1∗ | DBA | ICUS | — | Sister with primary myelofibrosis | — |
| PV2663 | 29/F | GATA2 | GATA2-deficiency syndrome | ICUS | Hashimoto thyroiditis and papillary carcinoma; chronic gastritis; obesity; early menarche | Paternal aunt with breast cancer and paternal grandfather with esophagus cancer; maternal grandmother with pancreatic cancer | — |
| PV2274 | 23/F | GATA2 | GATA2-deficiency syndrome | AML | Long-lasting peripheral blood cytopenia; recurrent respiratory infections | — | — |
| PV1264 | 24/F | GATA2 | GATA2-deficiency syndrome | MDS-MLD | — | Grandfather with multiple myeloma | — |
| PV2661 | 45/M | NF1 | NF | CMML-0 | Moderate-to-severe cognitive deficit, aortic stenosis and hypertension, short stature; GIST in imatinib therapy | — | — |
| PV2662 | 44/F | PTPN11 | NS | ICUS | Short stature, amenorrhea since age 25, hepatic fibrosis, portal hypertension and esophageal varices | — | — |
| PV2664 | 38/M | RPS26 | DBA | ICUS | Steroid diabetes | Paternal grandmother died of lung cancer | — |
| PV1774 | 52/F | RUNX1∗ | RUNX1-related FPD | MDS/MPN-U | Essential tremor since age 29; low count platelets since 1985 | Brother with CMML; cousin died of leukemia | Father died of lung fibrosis |
| PV2117 | 24/F |
SBDS∗ SBDS∗ |
SDS | ICUS | Short stature, intellectual disability/low IQ; pancreatic insufficiency; knee chondropathy | — | Brother with isolated neutropenia |
ALL, acute lymphoblastic leukemia; APCAs, antiparietal cell antibodies; CMML, chronic myelomonocytic leukemia; DBA, Diamond-Blackfan anemia; F, female; FA, Fanconi anemia; FPD, familial platelet disorder; GIST, gastrointestinal stromal tumor; ID, identification number; M, male; MDS-EB, MDS with excess blasts; MDS-MLD, myelodysplastic syndrome with multilineage dysplasia; MDS/MPN-U, MDS/myeloproliferative neoplasm unclassifiable; NF, neurofibromatosis; NS, Noonan syndrome; SDS, Shwachman-Diamond syndrome; SCN, severe congenital neutropenia.
The same FANCA variant was detected in heterozygosity in the son who was not affected. The same GATA1 variant was detected in a sister with primary myelofibrosis. The same RUNX1 variant was detected in the brother affected with CMML. Both SBDS variants were detected in the brother with isolated neutropenia.
Of 27 patients (48%) with causative genotype, 13 also carried oncogenic somatic variants and/or cytogenetic abnormalities (Figure 1C; Table 2). No significantly higher prevalence of somatic lesions (48% vs 45%; P = .44) or higher number of somatic lesions per individual (P = .73) was found in patients with causative genotype than that in those without germ line mutation. Overall, genes of the histone/chromatin modification pathway were the most commonly mutated (35%), followed by genes involved in cohesin complex (18%), DNA methylation (12%), transcription regulation (6%), signal transduction (6%), and RNA splicing (6%), without significantly different prevalence compared with patients without germ line mutation. Somatic mutations in DDX41 accounted for 17% of all the observed somatic variants, almost invariably in combination with a predisposing germ line hit in the same gene.
The most common predisposition syndrome/disorder was DDX41-associated predisposition, detected in 12 out of 402 (3%) patients with hypocellular bone marrow (supplemental Figure 2). These accounted for 10 (7 with MDS with excess blasts, 2 with MDS with multilineage dysplasia, and 1 with AML) of 173 patients (5.8%) receiving a diagnosis of myeloid neoplasm, whereas the remaining 2 received a diagnosis of ICUS/CCUS (Figure 1D). The median age at the time of diagnosis was 63 years (Figure 2D), with a significantly higher male prevalence (83%; P < .001). Only 1 patient had a family history of solid tumors, and the same patient had a colorectal cancer. Three of the 12 (25%) patients with P/LP germ line DDX41 mutation carried a second somatic hit in the same gene, whereas overall 5 of 8 observed variants (DDX41 p.[M1I], p.[K102fs], p.[I207T], p.[G218D], and p.[Y259H]) were found in our study and/or previously reported as associated with somatic DDX41 hit.21 Two patients had somatic mutation in ASXL1 (17%), in combination with ATRX and BCOR in 1 case, and STAG2 in the other, whereas 1 additional patient harbored a somatic hit in SF3B1. Of the 10 patients receiving a diagnosis of myeloid malignancy, 7 showed a normal karyotype.
Clinical correlates of predisposition syndromes/disorders in adults with suspected myeloid neoplasm
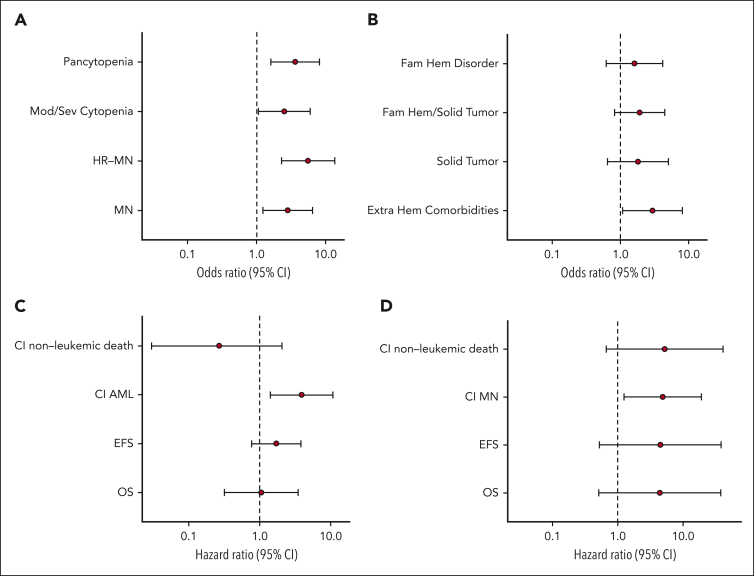
Patients with a congenital syndrome/disorder were younger at the time of the clinical onset than those without mutations (median age, 51 vs 57 years; P = .03) (Figure 2A-B). When excluding patients with DDX41 mutation, known to have a late-onset hematologic disorder, the age at diagnosis of patients with other congenital syndromes/disorders was even lower than that of patients without germ line mutations (median age, 38 vs 57 years; P < .001; Figure 2C). A genotype consistent with a congenital syndrome/disorder was associated with a significantly higher risk of receiving a diagnosis of myeloid neoplasm (odds ratio [OR], 2.83; P = .014) as well as of receiving a diagnosis of high-risk myeloid neoplasm (MDS with excess blasts type 2 or AML) (OR, 5.58; P < .001). In addition, it was associated with a significantly higher risk of neutropenia (OR, 3.51; P = .014), moderate-to-severe neutropenia (OR, 3.51; P = .002), multiple cytopenias (OR, 3.63; P = .002), and more severe cytopenia (OR, 2.51; P = .036; Figure 3A) as well as with a greater prevalence of extrahematologic comorbidities (OR, 2.96; P = .037; Figure 3B). Conversely, no significant association was observed between congenital syndrome/disorder and medical history of multiple tumors or family history of hematologic/solid tumors (P = .26 and P = .13, respectively; Figure 3B).
Figure 3.
Clinical correlates and outcomes of germ line mutations causative of a congenital syndrome or disorder. (A) Forest plot of the ORs for myeloid neoplasm (MN), high-risk MN, moderate-to-severe cytopenia, and pancytopenia in patients harboring germ line mutations identified as causative for a congenital syndrome or disorder (S/D). (B) Forest plot of the ORs for extrahematologic comorbidities, solid tumor(s), familiarity for hematologic/solid tumors (Fam Hem/Solid Tumor), or nonmalignant hematologic disorders (Fam Hem Disorder) in patients with S/D. (C) Forest plot of the HRs for OS, event-free survival, and cumulative incidence of AML or nonleukemic death in patients with S/D receiving a diagnosis of MN. (D) Forest plot of the HRs for OS, event-free survival, and cumulative incidence of MN or nonleukemic death in S/D patients receiving a diagnosis of nonneoplastic disorder. ORs and HRs are shown on a logarithmic scale with 95% confidence interval (CI).
In the whole cohort of patients, a genotype consistent with a congenital syndrome/disorder did not show a significantly reduced overall survival (OS) (hazard ratio [HR], 1.32; P = .59; supplemental Figure 3). These results were confirmed when patients with germ line DDX41 mutation were excluded from the analysis (HR, 1.79; P = .34). Conversely, this genotype was associated with significantly reduced event-free survival (HR, 2.18; P = .041) (supplemental Figure 4).
A genotype consistent with a congenital syndrome or disorder was significantly associated with increased cumulative incidence of progression into AML, estimated with a competing risk approach either in the whole cohort (HR, 4.90; P = .002) or in patients with a diagnosis of myeloid neoplasm (HR, 3.92; P = .008; Figure 3C; supplemental Figure 5).
Finally, in patients receiving a diagnosis of nonneoplastic disorder, a congenital syndrome/disorder was associated with a cumulative incidence of myeloid neoplasm (HR, 4.87; P = .023), whereas no significant effect on OS, event-free survival, or cumulative incidence of nonleukemic death was observed (Figure 3D).
Prevalence of carriers of recessive predisposition to myeloid neoplasms and penetrance for myeloid neoplasm
A unique heterozygous P/LP mutation in genes associated with autosomal recessive disorders was identified in 10 out of 402 (2.5%) cases (Figure 1A). Among these 10 patients, the most common mutated gene was SBDS (n = 5), followed by FANCD1 (BRCA2) (n = 2), CSF3R (n = 1), DNAJC21 (n = 1), and FANCD2 (n = 1) (Figure 1B; Table 3; supplemental Table 3).
Within this group, heterozygous CSF3R and FANCD1 (BRCA2) variants were recently suggested as possibly associated with predisposition to hematologic malignancies.34, 35, 36
Collectively, 4 patients carrying germ line mutations (40%) received a diagnosis of myeloid neoplasm, whereas the remaining 6 received a provisional diagnosis of ICUS/CCUS (Figure 1C). The median age at the time of diagnosis was 47 years (range, 25-70 years), with a male preponderance (7 of 10 patients). Patients who carried mutations tended to be younger at the time of the clinical onset than those without mutations (median age, 47 vs 57 years; P = .12) (Figure 2A-B) yet were mostly older than those with homozygosity/combined heterozygosity for the related syndrome/disorder (supplemental Figure 6).
Two patients had a family history of malignancy, whereas 4 had a personal history of multiple tumors. Five patients harbored concomitant somatic lesions, including 4 patients diagnosed with myeloid malignancy and 1 receiving a provisional diagnosis of CCUS. No significantly higher prevalence of somatic lesions or higher number of somatic lesions per individual was noticed in those who carried mutations compared with patients with wild-type genes (P = .5 and P = .6, respectively). RNA splicing genes were the most commonly affected (28%), followed by genes involved in DNA methylation (18%), signal transduction (18%), tumor suppression (18%), histone/chromatin modification (9%), and transcription regulation (9%).
Carrying a germ line mutation in genes associated with autosomal recessive disorders was not associated with reduced OS in the whole cohort (P = .28) nor in patients receiving a diagnosis of myeloid neoplasm (P = .55). In addition, no significantly higher prevalence of more severe/multiple cytopenia and myeloid neoplasm or higher-risk myeloid neoplasm were found in patients carrying mutations in genes associated with autosomal recessive disorders (supplemental Figure 7). Finally, no significantly higher cumulative incidence of myeloid neoplasm was noticed in patients with mutations who did not receive a diagnosis of malignant disorder at the time of first assessment, and no significantly increased cumulative incidence of AML was noticed in patients receiving a diagnosis of myeloid neoplasm.
Germ line VUS in genes associated with predisposition to myeloid neoplasms
We identified 111 germ line VUSs in 38 genes associated with predisposition to myeloid neoplasms, in 88 out of 402 patients (21.9%) (Figure 1A; supplemental Figure 1; supplemental Table 4).
Among these VUSs, we detected 13 variants that failed ACMG/AMP P/LP classification but presented multiple levels of evidence for a pathogenic effect, including a total population variant frequency <0.001 in gnomAD,37 a higher number of damaging in silico functional predictions, and the absence of ACMG/AMP benign criteria. In addition, this evidence was supported by a suggestive patient’s hematologic and/or extrahematologic phenotype, family history, and/or scientific literature. Overall, 12 patients harbored such variants, either in genes associated with autosomal dominant predisposition to hematologic malignancies (including TERC/TERT, n = 4; DDX41, n = 2; and CBL, GATA2, RPL11, and SOS1, n = 1), or in homozygosity/compound heterozygosity in genes associated with autosomal recessive syndromes (FANCA, n = 2) (supplemental Table 4).
We identified 8 patients (2%) harboring germ line VUSs in SAMD9 or SAMD9L. Almost all the detected SAMD9/SAMD9L germ line variants localized at the N-terminal and central region of both genes, consistently with mutations previously reported in adult patients (supplemental Results; supplemental Figure 8).38
In addition, germ line VUSs in DHX34, recently reported as a novel locus involved in inherited forms of myeloid neoplasm,39 were detected in 13 patients (3.2%) (supplemental Results; supplemental Figure 9). Overall, we identified 11 missense variants and 2 truncating variants, DHX34 p.(E627∗) and p.(H1086fs), not previously described (supplemental Results).
Finally, we identified 9 patients harboring CSF3R p.(E808K) (n = 6) and MPL p.(K39N) (n = 3) variants, previously reported as risk factors for high-risk MDS and thrombocytosis, respectively.40, 41, 42, 43
Diagnostic implications of germ line mutations in adult patients with suspected myeloid neoplasm
We aimed to investigate the implications of germ line mutations in the diagnostic work-up of adult patients with suspected myeloid neoplasm. In fact, germ line mutations may be associated with changes in bone marrow cells that overlap with dysplastic features, irrespective of whether the patient has a myeloid malignancy, thus complicating the diagnosis.3,25 Therefore, we investigated the positive and negative predictive values of somatic mutations for identifying individuals with a myeloid neoplasm in the context of a germ line predisposition syndrome/disorder. Among the patients with germ line predisposition syndromes/disorders, 6 patients receiving a diagnosis of myeloid neoplasm did not have detectable somatic genetic lesions. Overall, in this context, having ≥1 somatic mutations had a positive predictive value of 92.3% for the diagnosis of myeloid neoplasm. Conversely, the absence of somatic genetic lesions had a negative predictive value of 57.1% for excluding a diagnosis of myeloid neoplasm. When limiting the analysis to low-risk myeloid neoplasms, which are associated with the highest diagnostic uncertainty based on standard morphologic criteria, the negative predictive value was 80%.
Then, we aimed at defining demographic and clinical variables that could be useful to identify adult patients with cytopenia and bone marrow hypocellularity with higher likelihood of having underlying germ line mutation, and, accordingly, to inform germ line genetic testing. Clinical presentation with pancytopenia (OR, 3.63; P = .002) as well as the presence of extrahematologic comorbidities (OR, 2.96; P = .037) was predictive of a genotype causative of congenital syndrome or disorder. Conversely, other variables potentially suggestive of an underlying germ line predisposition, including a family history of hematologic or solid cancer, as well as a personal history of multiple tumors, did not show significant association with a genotype consistent with congenital syndrome or disorder (P values ranging from .13 to .32). We applied multinomial regression models including demographic, clinical, and family history variables to investigate the extent to which the presence of a germ line predisposition can be inferred from clinical phenotype in our population of adult patients with hypocellular bone marrow (supplemental Figure 10). The combination of the relevant clinical variables resulted in a maximum pseudo-R2 of 0.14, suggesting that the prediction of patients with germ line predisposition based on demographic, clinical, and family history, in a population of adult patients, carrying disorders with mild clinical expressivity and incomplete penetrance, is limited.
Discussion
Germ line variants predisposing to myeloid neoplasms are increasingly recognized in patients with MDS or AA.1,9,15 Systematic studies of the frequency of germ line predisposition variants, their penetrance and clinical expressivity in adults with apparently sporadic myeloid neoplasms, and reliable clinical indicators driving appropriate genetic testing are limited.11,12 Bone marrow hypocellularity has been reported as a common feature in adult patients with late onset of congenital BMF syndromes,14,15 and underlying unrecognized inherited conditions likely contribute to the challenging classification of disorders with hypocellular bone marrow.22 In this work, we studied a large cohort of well-annotated consecutive adult patients with cytopenia and hypocellular bone marrow by means of germ line and somatic targeted sequencing to estimate the prevalence of germ line predisposition variants and their clinical relevance.
We identified a prevalence of 6.7% of germ line predisposition variants in unselected individuals with hypocellular bone marrow. The most common mutated gene was DDX41, which accounted for 6% of apparently sporadic MDS/AML. In line with previous reports, these patients had a late-onset neoplasm in the absence of an extrahematologic phenotype or significant family history of cancer.17, 18, 19, 20 Notably, although most of these patients received a diagnosis of myeloid neoplasm, 1 of them received the diagnosis of CCUS, whereas 1 additional patient was diagnosed with ICUS, suggesting that the germ line variant may result in a mild hematologic phenotype preceding its progression to a malignancy, as previously hypothesized.17 Only 3 patients with germ line P/LP DDX41 variants in our series were found to have a second somatic hit in the same gene. However, this association was proven in 5 of 8 observed variants based on our study and/or previous reports. In addition, it must be acknowledged that our study enrolled patients with unexplained cytopenia, thus including earlier disease stages compared with previous studies in which patients with overt myeloid neoplasms were selected.21
Identifying patients with underlying genetic predisposition has relevant clinical implications. Previous studies reported that patients with MDS with an underlying undiagnosed Shwachman-Diamond syndrome or telomere disease showed dismal outcome after allogeneic hematopoietic stem cell transplantation.11,12 We found that in patients receiving a diagnosis of myeloid neoplasm, carrying a germ line predisposition was associated with more severe bone marrow failure, higher-risk disease, and increased risk of progression to AML, compared with patients without a germ line predisposition. In addition, in patients without a diagnosis of myeloid neoplasm, an underlying genetic predisposition was associated with a significantly higher cumulative incidence of its progression to myeloid malignancy.
The findings of our study confirm that bone marrow hypocellularity is a setting with a relevant prevalence of BMF-associated germ line variants. It must be acknowledged that this inclusion criterion might have resulted in a selection of cases within predisposing conditions to myeloid neoplasms. Although a direct comparison with cases with normo- or hypercellular bone marrow was outside the scope of this study, our results suggest that age-adjusted hypocellularity is a potential indicator for genetic testing, in particular in younger adults. In turn, the recognition of an underlying genetic disorder may contribute to unveiling a previously unaccounted source of heterogeneity in the diagnostic work-up of adult patients with cytopenia who display a reduced age-adjusted bone marrow cellularity. This evidence is even more relevant after the recent recognition of hypoplastic MDS as a distinct disease subtype.4
Notably, 30% of patients carrying germ line mutations causative of a congenital syndrome or disorder received a diagnosis of ICUS without evidence of myeloid neoplasm or somatic clonal evolution, supporting a screening for inherited BMF also in those adult patients with hypocellular bone marrow, in whom a diagnosis of myeloid neoplasm has been ruled out. Furthermore, a fraction of patients carrying germ line predisposition variants received a diagnosis of myeloid neoplasm without eventual proof of somatic clonal evolution, confirming that conventional morphologic criteria in this setting may be inadequate to support an accurate diagnosis of malignant vs nonmalignant hematologic phenotype.22 In addition, detecting a germ line mutation appears to be critical to guiding somatic mutation profiling, under the selective pressure of the underlying genetic lesion.28,32,44
A fraction of patients carried germ line variants in genes associated with autosomal recessive disorders. In our cohort, this was not associated with reduced survival. In addition, no significantly higher prevalence of somatic mutations or number of somatic mutations per individual were noted compared with patients with wild-type genes. Despite the limited sample size, collectively, these data suggest that heterozygous germ line mutations in genes associated with disorders reported as autosomal recessive based on clinical phenotype are not associated with higher risk of developing a myeloid neoplasm or more severe bone marrow failure.
In our study, we found a high prevalence of VUS in genes predisposing to myeloid neoplasms. Among these, we found 2% of cases harboring germ line loss-of-function mutations in SAMD9 or SAMD9L, almost invariably localized at the N-terminal and central region of both genes, consistently with the variants previously reported in adult patients with either MDS or BMF.38 Although, currently, these variants are more sensibly interpreted as being of uncertain significance, our results are consistent with the available evidence and support a potential role in predisposition to myeloid neoplasm in adult patients with disorders within the spectrum of BMF/MDS. In addition, in our cohort, we also found a fraction of patients carrying missense or truncating variants in DHX34, recently reported as a novel locus involved in inherited forms of hypoplastic MDS/AML.39,45,46 In vitro studies to functionally validate the effect of these variants are ongoing.
Overall, these results highlight that, whereas applying robust criteria for classification of germ line variants is unquestionable,30,31,47,48 generating appropriate evidence through functional studies is warranted for further increasing the sensitivity in capturing and correctly classifying relevant germ line variants in the clinical setting.49
The results of our study also underline the challenges of using genetic testing in adult patients with apparently sporadic suspected myeloid neoplasms. In fact, late clinical onset in adulthood likely subtends a selection of disorders and variants with incomplete penetrance and/or mild clinical expressivity, hampering a clinically driven screening. Only a fraction of patients reported a family history of hematologic or extrahematologic malignancy or showed an extrahematologic phenotype or other independent cancer fostering suspicion of a germ line disorder.50 Accordingly, combining all the potentially informative clinical variables resulted in a very limited predictive value for an underlying genetic predisposition. In addition, the interpretation of variants in this setting introduces an additional level of complexity, because the absence of clear genotype-phenotype associations and an often-silent family history may potentially result in a reduced sensitivity in capturing clinically meaningful variants.
In conclusion, the findings of this study unveil the spectrum, prevalence, and clinical expressivity of germ line predisposition variants in an unselected cohort of adult patients with cytopenia and bone marrow hypocellularity. Identifying an underlying germ line genetic predisposition has relevant clinical implications, because patients with myeloid neoplasm carrying predisposition variants show more severe disease and increased risk of leukemic progression. These results also suggest that age-adjusted hypocellularity may represent an indication for germ line genetic testing in adults, although facing a spectrum of disorders and variants with incomplete penetrance and/or mild clinical expressivity complicates the development of clinically informed strategies for genetic testing.
Conflict-of-interest disclosure: E.R. holds shares of enGenome SRL, a bioinformatics company. The remaining authors declare no competing financial interests.
Acknowledgments
This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (Investigator Grant #20125; AIRC 5×1000 project #21267); Cancer Research UK, Fundacion Cientifica–Asociacion Espanola Contra el Cancer, Spain, and AIRC under the International Accelerator Award Program (project #C355/A26819 and #22796); and Fondazione Cariplo (project #2017-1910).
Authorship
Contribution: E.M. performed mutation analysis and contributed to study design and interpretation of the data; E. Bono collected clinical data and samples and contributed to study design, statistical analysis, and interpretation of the data; A.G. and M.S. analyzed sequencing data; C.E., J.F., N.F., V.C., and E. Boveri collected clinical data and samples; V.V.F., E.R., and S.P. performed biostatistics, bioinformatic analysis, and data management; M.C. contributed to study design and interpretation of the data; L.M. designed the study, performed statistical analysis, and wrote the manuscript; and all authors contributed to manuscript preparation and approved its content.
Footnotes
∗E.M. and E. Bono contributed equally to this study.
Deidentified data are available on request from the corresponding author, Luca Malcovati (luca.malcovati@unipv.it).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:1391–1405. [Google Scholar]
- 2.Godley LA, Shimamura A. Genetic predisposition to hematologic malignancies: management and surveillance. Blood. 2017;130(4):424–432. doi: 10.1182/blood-2017-02-735290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian RP, et al. International Consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastor V, Hirabayashi S, Karow A, et al. Mutational landscape in children with myelodysplastic syndromes is distinct from adults: specific somatic drivers and novel germline variants. Leukemia. 2017;31(3):759–762. doi: 10.1038/leu.2016.342. [DOI] [PubMed] [Google Scholar]
- 6.Wlodarski MW, Hirabayashi S, Pastor V, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127(11):1387–1397. doi: 10.1182/blood-2015-09-669937. quiz 518. [DOI] [PubMed] [Google Scholar]
- 7.Guidugli L, Johnson AK, Alkorta-Aranburu G, et al. Clinical utility of gene panel-based testing for hereditary myelodysplastic syndrome/acute leukemia predisposition syndromes. Leukemia. 2017;31(5):1226–1229. doi: 10.1038/leu.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AL, Churpek JE, Malcovati L, Dohner H, Godley LA. Recognition of familial myeloid neoplasia in adults. Semin Hematol. 2017;54(2):60–68. doi: 10.1053/j.seminhematol.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Kraft IL, Godley LA. Identifying potential germline variants from sequencing hematopoietic malignancies. Blood. 2020;136(22):2498–2506. doi: 10.1182/blood.2020006910. [DOI] [PubMed] [Google Scholar]
- 10.The University of Chicago Hematopoietic Malignancies Cancer Risk Team How I diagnose and manage individuals at risk for inherited myeloid malignancies. Blood. 2016;128(14):1800–1813. doi: 10.1182/blood-2016-05-670240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536–547. doi: 10.1056/NEJMoa1611604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly CR, Myllymäki M, Redd R, et al. The clinical and functional effects of TERT variants in myelodysplastic syndrome. Blood. 2021;138(10):898–911. doi: 10.1182/blood.2021011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feurstein SK, Trottier AM, Estrada-Merly N, et al. Germline predisposition variants occur in myelodysplastic syndrome patients of all ages. Blood. 2022;140(24):2533–2548. doi: 10.1182/blood.2022015790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota Y, Zawit M, Durrani J, et al. Significance of hereditary gene alterations for the pathogenesis of adult bone marrow failure versus myeloid neoplasia. Leukemia. 2022;36(12):2827–2834. doi: 10.1038/s41375-022-01729-4. [DOI] [PubMed] [Google Scholar]
- 15.Feurstein S, Churpek JE, Walsh T, et al. Germline variants drive myelodysplastic syndrome in young adults. Leukemia. 2021;35(8):2439–2444. doi: 10.1038/s41375-021-01137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluteau O, Sebert M, Leblanc T, et al. A landscape of germ line mutations in a cohort of inherited bone marrow failure patients. Blood. 2018;131(7):717–732. doi: 10.1182/blood-2017-09-806489. [DOI] [PubMed] [Google Scholar]
- 17.Sebert M, Passet M, Raimbault A, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134(17):1441–1444. doi: 10.1182/blood.2019000909. [DOI] [PubMed] [Google Scholar]
- 18.Lewinsohn M, Brown AL, Weinel LM, et al. Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood. 2016;127(8):1017–1023. doi: 10.1182/blood-2015-10-676098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polprasert C, Schulze I, Sekeres MA, et al. Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 2015;27(5):658–670. doi: 10.1016/j.ccell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Brown S, Williams M, et al. The genetic landscape of germline DDX41 variants predisposing to myeloid neoplasms. Blood. 2022;140(7):716–755. doi: 10.1182/blood.2021015135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makishima H, Saiki R, Nannya Y, et al. Germ line DDX41 mutations define a unique subtype of myeloid neoplasms. Blood. 2023;141(5):534–549. doi: 10.1182/blood.2022018221. [DOI] [PubMed] [Google Scholar]
- 22.Bono E, McLornan D, Travaglino E, et al. Clinical, histopathological and molecular characterization of hypoplastic myelodysplastic syndrome. Leukemia. 2019;33(10):2495–2505. doi: 10.1038/s41375-019-0457-1. [DOI] [PubMed] [Google Scholar]
- 23.Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943–2964. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itzykson R, Fenaux P, Bowen D, et al. Diagnosis and treatment of chronic myelomonocytic leukemias in adults: recommendations from the European Hematology Association and the European LeukemiaNet. Hemasphere. 2018;2(6):e150. doi: 10.1097/HS9.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncavage EJ, Bagg A, Hasserjian RP, et al. Genomic profiling for clinical decision making in myeloid neoplasms and acute leukemia. Blood. 2022;140(21):2228–2247. doi: 10.1182/blood.2022015853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]
- 27.Valent P, Orazi A, Steensma DP, et al. Proposed minimal diagnostic criteria for myelodysplastic syndromes (MDS) and potential pre-MDS conditions. Oncotarget. 2017;8(43):73483–73500. doi: 10.18632/oncotarget.19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malcovati L, Cazzola M. The shadowlands of MDS: idiopathic cytopenias of undetermined significance (ICUS) and clonal hematopoiesis of indeterminate potential (CHIP) Hematology Am Soc Hematol Educ Program. 2015;2015(1):299–307. doi: 10.1182/asheducation-2015.1.299. [DOI] [PubMed] [Google Scholar]
- 29.Padron E, Ball MC, Teer JK, et al. Germ line tissues for optimal detection of somatic variants in myelodysplastic syndromes. Blood. 2018;131(21):2402–2405. doi: 10.1182/blood-2018-01-827881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X, Feurstein S, Mohan S, et al. ClinGen myeloid malignancy variant curation expert panel recommendations for germline RUNX1 variants. Blood Adv. 2019;3(20):2962–2979. doi: 10.1182/bloodadvances.2019000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malcovati L, Galli A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371–3378. doi: 10.1182/blood-2017-01-763425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli A, Todisco G, Catamo E, et al. Relationship between clone metrics and clinical outcome in clonal cytopenia. Blood. 2021;138(11):965–976. doi: 10.1182/blood.2021011323. [DOI] [PubMed] [Google Scholar]
- 34.Trottier AM, Druhan LJ, Kraft IL, et al. Heterozygous germ line CSF3R variants as risk alleles for development of hematologic malignancies. Blood Adv. 2020;4(20):5269–5284. doi: 10.1182/bloodadvances.2020002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner JE, Tolar J, Levran O, et al. Germline mutations in BRCA2: shared genetic susceptibility to breast cancer, early onset leukemia, and Fanconi anemia. Blood. 2004;103(8):3226–3229. doi: 10.1182/blood-2003-09-3138. [DOI] [PubMed] [Google Scholar]
- 36.Macedo GS, Alemar B, Ashton-Prolla P. Reviewing the characteristics of BRCA and PALB2-related cancers in the precision medicine era. Genet Mol Biol. 2019;42(suppl 1):215–231. doi: 10.1590/1678-4685-GMB-2018-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamshad MJ, Ng SB, Bigham AW, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 38.Nagata Y, Narumi S, Guan Y, et al. Germline loss-of-function SAMD9 and SAMD9L alterations in adult myelodysplastic syndromes. Blood. 2018;132(21):2309–2313. doi: 10.1182/blood-2017-05-787390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rio-Machin A, Vulliamy T, Hug N, et al. The complex genetic landscape of familial MDS and AML reveals pathogenic germline variants. Nat Commun. 2020;11(1):1044. doi: 10.1038/s41467-020-14829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfler A, Erkeland SJ, Bodner C, et al. A functional single-nucleotide polymorphism of the G-CSF receptor gene predisposes individuals to high-risk myelodysplastic syndrome. Blood. 2005;105(9):3731–3736. doi: 10.1182/blood-2004-06-2094. [DOI] [PubMed] [Google Scholar]
- 41.Liongue C, Ward AC. Granulocyte colony-stimulating factor receptor mutations in myeloid malignancy. Front Oncol. 2014;4:93. doi: 10.3389/fonc.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moliterno AR, Williams DM, Gutierrez-Alamillo LI, Salvatori R, Ingersoll RG, Spivak JL. Mpl Baltimore: a thrombopoietin receptor polymorphism associated with thrombocytosis. Proc Natl Acad Sci U S A. 2004;101(31):11444–11447. doi: 10.1073/pnas.0404241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleyrat C, Girard R, Choi EH, et al. Gene editing rescue of a novel MPL mutant associated with congenital amegakaryocytic thrombocytopenia. Blood Adv. 2017;1(21):1815–1826. doi: 10.1182/bloodadvances.2016002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armes H, Rio-Machin A, Krizsan S, et al. Acquired somatic variants in inherited myeloid malignancies. Leukemia. 2022;36(5):1377–1381. doi: 10.1038/s41375-022-01515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivera OD, Mallory MJ, Quesnel-Vallieres M, et al. Alternative splicing redefines landscape of commonly mutated genes in acute myeloid leukemia. Proc Natl Acad Sci U S A. 2021;118(15) doi: 10.1073/pnas.2014967118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hug N, Aitken S, Longman D, et al. A dual role for the RNA helicase DHX34 in NMD and pre-mRNA splicing and its function in hematopoietic differentiation. RNA. 2022;28(9):1224–1238. doi: 10.1261/rna.079277.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelb BD, Cave H, Dillon MW, et al. ClinGen's RASopathy Expert Panel consensus methods for variant interpretation. Genet Med. 2018;20(11):1334–1345. doi: 10.1038/gim.2018.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fortuno C, Lee K, Olivier M, et al. Specifications of the ACMG/AMP variant interpretation guidelines for germline TP53 variants. Hum Mutat. 2021;42(3):223–236. doi: 10.1002/humu.24152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malcovati L. The journey of a thousand miles begins with 1 step. Blood. 2021;138(10):824–826. doi: 10.1182/blood.2021012304. [DOI] [PubMed] [Google Scholar]
- 50.Singhal D, Hahn CN, Feurstein S, et al. Targeted gene panels identify a high frequency of pathogenic germline variants in patients diagnosed with a hematological malignancy and at least one other independent cancer. Leukemia. 2021;35(11):3245–3256. doi: 10.1038/s41375-021-01246-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.