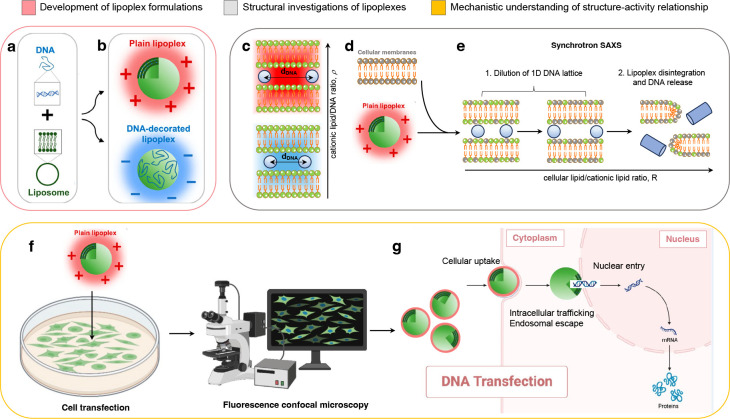
Figure 2.
The optimization of lipoplex formulations involved a comprehensive understanding of their mechanism of formation, structure, and structure–activity relationship. (a) When negatively charged DNA chains interact with positively charged CLs, an equal number of positive and negative Manning counterions are released, resulting in a gain in translational entropy. The isoelectric point is identified as the most thermodynamically favored state of lipoplexes due to its maximal entropic contribution to free energy. (b) Isoelectric instability of lipoplexes allows the accommodation of additional DNA or lipid material, leading to negatively charged DNA-decorated and positively charged lipoplexes. Positively charged lipoplexes are efficient gene delivery systems, interacting effectively with oppositely charged cell membranes and yielding high transfection levels. (c) Synchrotron small-angle X-ray scattering (SAXS) revealed that lipoplex formation induces a topological transition in a lamellar phase structure composed of DNA monolayers between lipid bilayers. The 1D in-plane DNA lattice within this structure is relatively disordered, oscillating around equilibrium positions based on the cationic lipid/DNA ratio (ρ). (d) Studies propose that DNA release from lipoplexes occurs through charge neutralization by cellular lipids, which correlates with the interfacial curvature of the mesoscopic structures resulting from lipoplex–cellular lipid interaction. Lipoplexes that easily transform into nonlamellar phases when mixed with cellular lipids exhibit high transfection efficiency. (e) Cellular lipids progressively neutralize the lipid membrane of lipoplexes, leading to the increased distance between DNA molecules based on the cellular lipid/cationic lipid ratio. Structural evolution rates and susceptibility to destabilization by anionic lipids vary, influencing the dissociation of DNA from the lipid and correlating with transfection efficiency. (f) Transfection efficiency studies coupled with fluorescence confocal microscopy investigations elucidate the barriers to lipoplex-mediated transfection at the single-cell level. (g) Investigations explore cellular uptake, intracellular trafficking, endosomal escape, and nuclear entry of lipoplexes, providing mechanistic understanding for the development of multifunctional envelope-type nanodevices (MEND) and multicomponent envelope-type nanoparticle systems (MENS). A portion of the figure (panel b) was readapted from ref (16). Copyright 2022 American Chemical Society. Cartoons were created using Biorender.com.