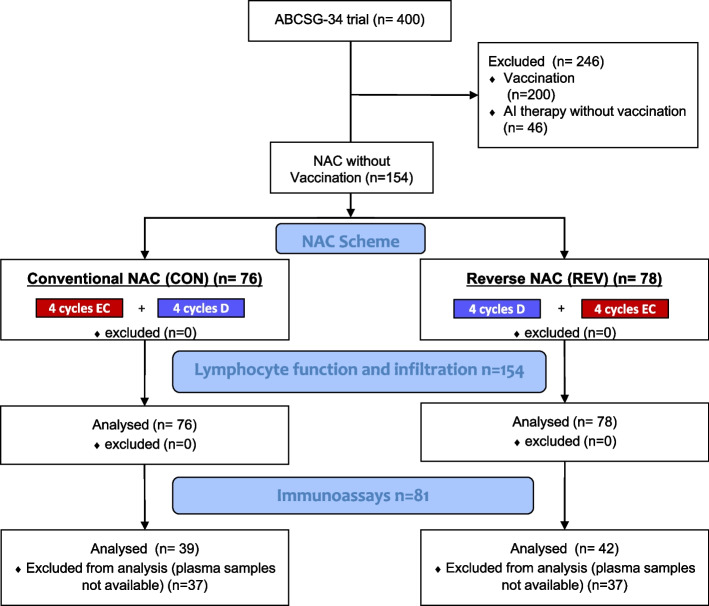
Fig. 1.
Patient selection diagram. This study was based on the ABCSG-34 trial which included a total of 400 patients. Half of them got a vaccination, which was out of the scope of the current study, and they were therefore excluded. Additionally, we excluded non-vaccinated patients treated with aromatase inhibitors (AI). The remaining 154 patients were randomly assigned to two treatment arms with different sequences of neoadjuvant chemotherapy (NAC): the conventional sequence (CON, n = 76) with 4 cycles of EC followed by 4 cycles of D and the reverse sequence (REV, n = 78) with first D then EC. From this collective (n = 154) we determined lymphocyte function (i.e. PHA-induced IFNγ production of circulating lymphocytes) and infiltration (i.e. TILs). Immunoassays for characterization of lymphocyte subpopulation (e.g. CD3 and CD19) and for quantification of soluble immune mediators were started later and were therefore only determined in a subgroup (n = 81)