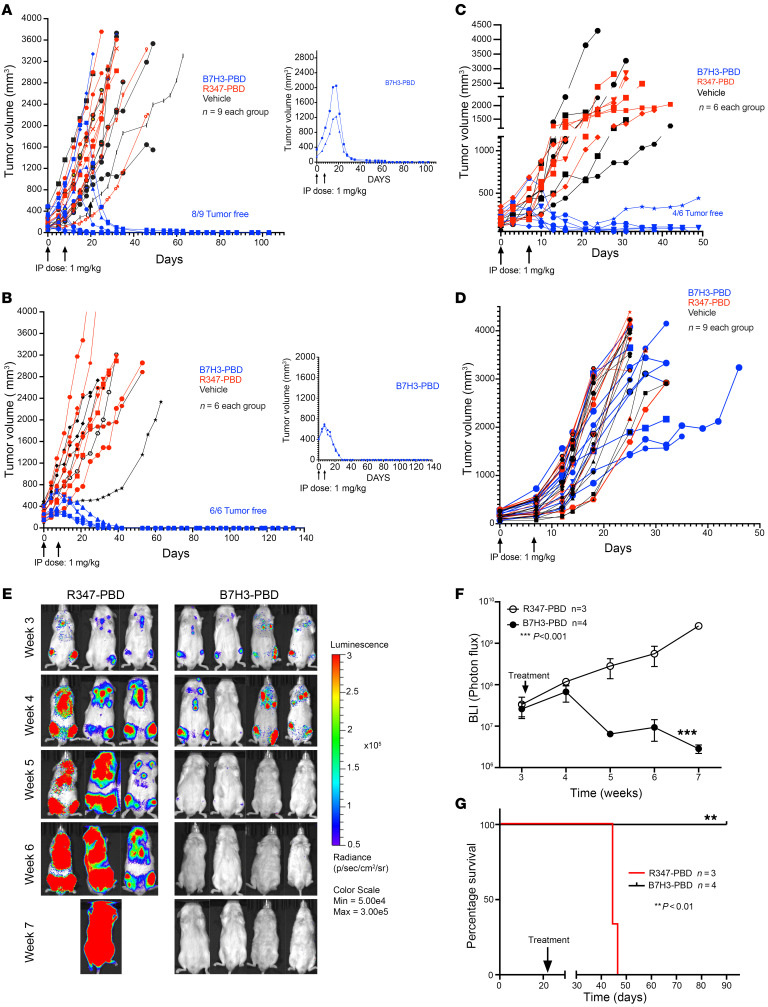
Figure 5. Prostate cancer organoid-derived biomarkers predict in vivo tumor responses in preclinical trials of the B7H3-PBD-ADC.
(A–D) Tumor response to B7H3-PBD-ADC (1 mg/kg), R347-PBD-ADC (1 mg/kg) or vehicle in the 4 selected LuCaP models based on identified biomarkers (A) LuCaP 145.2 (SCNPC phenotype; RB1loss, SLFN11+, IFN scoreHi), n = 9 / group. (B) LuCaP 136 (ARPC phenotype; RB1loss, SLFN11–, IFN scorelo), n = 6/ group. (C) LuCaP 77 (ARPC, RB1+, SLFN11+, IFN scoremedium) n = 6 /group. (D) LuCaP 167 (ARPC, RB1+, SLFN11–, IFN scorelo) n = 9 /group. Right panels for A and B display antitumor activity of B7H3-PBD-ADC in mice with large established tumors (145.2; > 1,000 mm3 and 136; > 650 mm3). Tumor volume measurements (mm3) are shown from the time of first treatment. Arrows indicate once weekly dose for 2 weeks. (E) BLI of LuCaP 136 metastases following treatment with B7H3-PBD-ADC (n = 4) or R347-PBD-ADC (n = 3). Mice were treated once weekly for 2 weeks. (F) Average BLI for treated mice from the time of first treatment. Means ± SD, 2-way ANOVA test; P < 0.001. (G) Kaplan-Meier survival analysis for the B7H3-PBD-ADC and R347-PBD-ADC treated LuCaP 136 metastases. Log-rank test (P < 0.01).