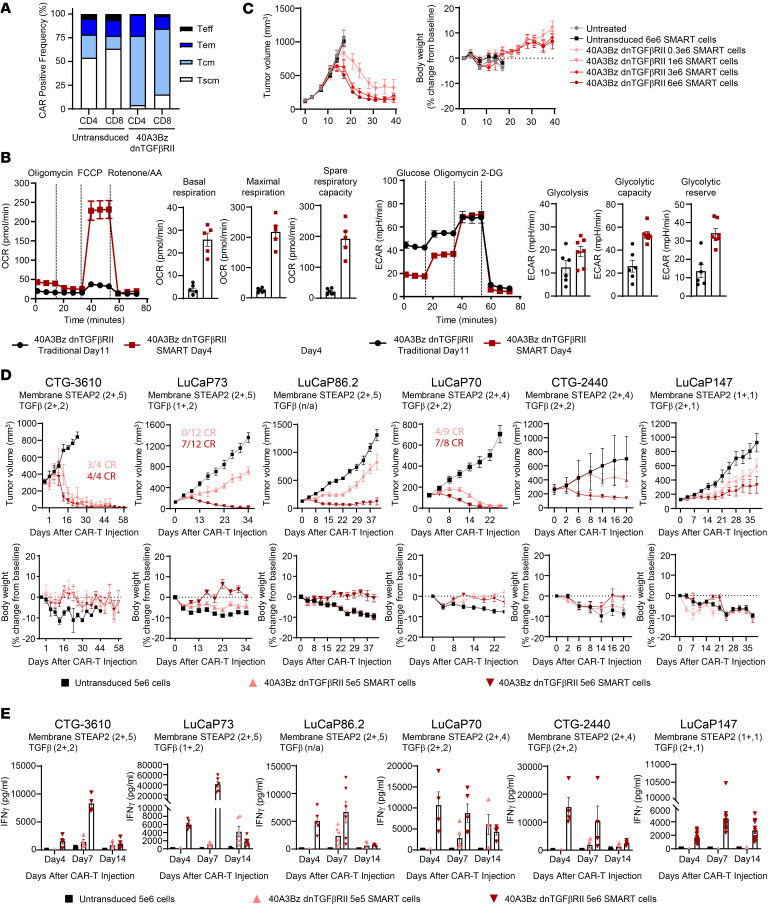
Figure 6. Effect of enhanced CAR-T manufacturing on antitumor activity.
(A) 40A3Bz dnTGFβRII CAR-Ts were manufactured according to the SMART process, and CAR positivity, activation, and phenotype of the cells were evaluated at expansion day 4 and compared with those of untransduced T cells from the same donor. (B) Bioenergetic profile of SMART (day 4) versus traditional (day 11) manufactured 40A3Bz dnTGFβRII CAR-Ts as determined by Seahorse analysis. OCR, oxygen consumption rate; ECAR, extracellular acidification rate. (C) 40A3Bz dnTGFβRII SMART CAR-Ts were dosed at 4 concentrations (0.3 × 106, 1 × 106, 3 × 106, and 6 × 106 CAR-positive cells) by tail vein injection in NSG MHC class I/II knockout mice implanted with 22Rv1 cells overexpressing TGF-β (n = 10). Tumor volumes and body weights were measured to 50 days after tumor implantation. (D) PDX fragments from frozen stocks of various prostate cancer PDX models were implanted into NSG MHC I/II knockout mice and randomized when tumor volumes for each model ranged from 125 to 250 mm3. Mice were dosed as described in C with 0.5 × 106 or 5 × 106 40A3Bz dnTGFβRII SMART CAR-Ts and compared with 5 × 106 untransduced SMART controls (n ranged from 4 to 12 depending on the model). The IHC data inset on each model represents the cell surface STEAP2 expression scoring. (E) Serum levels of IFN-γ across all PDX models described in D, determined by MSD (n = 4 or greater). Experiments are representative of 2 different donor CAR-Ts. Data in A–C are representative of multiple independent experiments prepared from 3 healthy donors, while D and E were performed once with material from 1 donor. All data represent mean ± SEM.