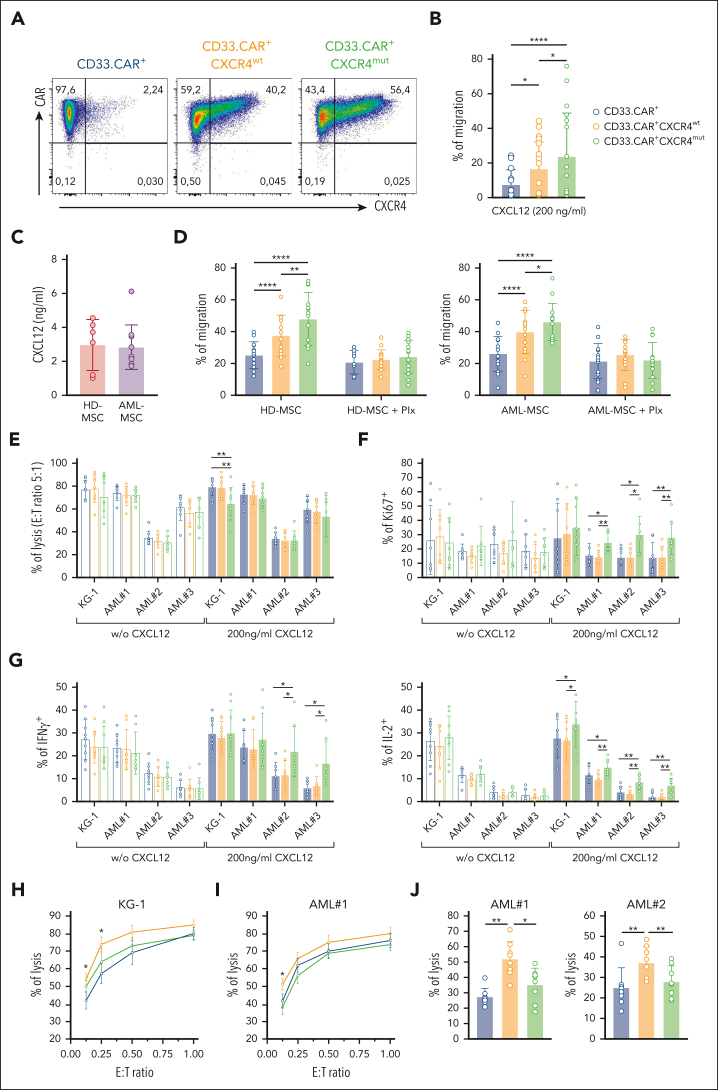
Figure 2.
CXCR4-overexpressing CD33.CAR-CIKs have improved migration capability toward CXCL12 and retain antitumor activity in vitro. Data are presented as individual values and the mean ± SD. (A) Representative flow cytometry plots showing CD33.CAR and CXCR4 expression on purified CD33.CAR+-, CD33.CAR+-CXCR4wt–, and CD33.CAR+-CXCR4mut–CIKs (supplemental Methods). (B) Percentage of migration of CXCR4-overexpressing CD33.CAR-CIKs in response to CXCL12 in transwell assays (n = 10 independent experiments using CAR-CIKs generated from 10 different donors; ∗∗∗∗P < .00001; ∗P = .015 for CD33.CAR+- vs CD33.CAR+-CXCR4wt–CIKs; ∗P = .0362 for CD33.CAR+-CXCR4wt– vs CD33.CAR+-CXCR4mut–CIKs, using paired t test). (C) CXCL12 levels measured in the culture supernatant of BM-derived mesenchymal stromal cells from healthy donors (HD-MSCs, n = 6 different donors) or from pediatric patients with AML (AML-MSCs, n = 10 different donors). (D) Percentage of migration of CXCR4-overexpressing CD33.CAR+-CIKs in response to culture supernatant of HD-MSCs (left) or AML-MSCs (right) in the absence or presence of plerixafor (Plx). For HD-MSCs: n = 12 experiments using CAR-CIKs generated from 6 different donors and supernatant samples from 6 different HD-MSCs; ∗∗∗∗P < .0001 and ∗∗P = .0056, using paired t test. For AML-MSCs: n = 14 experiments using CAR-CIKs generated from 6 different donors and supernatant samples from 10 different AML-MSCs; ∗∗∗∗P < .0001 and ∗P = .043, using paired t test. (E) Cytotoxicity (E:T ratio of 5:1) of CXCR4-overexpressing CD33.CAR+-CIKs against CD33+ KG-1 cell line and primary AML cells in the absence or presence of 200 ng/mL CXCL12 (for KG-1 with 200 ng/mL CXCL12: ∗∗P = .006 using paired t test). (F) Proliferation of CXCR4-overexpressing CD33.CAR+-CIKs in response to CD33+ KG-1 cell line and primary AML cells in the absence or presence of 200 ng/mL CXCL12 (for AML#1 with 200 ng/mL CXCL12: ∗P = .012; ∗∗P = .003; for AML#2 with 200 ng/mL CXCL12: ∗P = .012; for AML#3 with 200 ng/mL CXCL12: ∗∗P = .006 for CD33.CAR+-CXCR4mut– vs CD33.CAR+-CXCR4wt–CIKs; ∗∗P = .002 for CD33.CAR+-CXCR4mut– vs CD33.CAR+-CIKs. A paired t test was used). (G) Cytokine release of CXCR4-overexpressing CD33.CAR+-CIKs in response to CD33+ KG-1 cell line and primary AML cells in the absence or presence of 200 ng/mL CXCL12 (IFN-γ: ∗P = .012; IL-2: for KG-1 with 200 ng/mL CXCL12, ∗P = .029; for AML#1 with 200 ng/mL CXCL12, ∗P = .034 and ∗∗P = .005; for AML#2 and AML#3 with 200 ng/mL CXCL12, ∗∗P = .001. A paired t test was used.). For panels E, F, and G, n = 9 (for KG-1) and n = 8 (for primary AML cells) independent experiments using CAR-CIKs generated from different donors. (H-I) Quantification of (H) CD33+ KG-1 cell line and (I) primary AML cell lysis after 24 hours of coculture with CXCR4-overexpressing CD33.CAR+-CIKs at low E:T cell ratios, in the presence of 200 ng/mL CXCL12 (for KG-1 at E:T 0.25:1 and 0.125:1, ∗P = .03 for CD33.CAR+- vs CD33.CAR+-CXCR4wt–CIKs; for AML#1 at E:T 0.125:1, ∗P = .016 for CD33.CAR+-CXCR4wt– vs CD33.CAR+- and CD33.CAR+-CXCR4mut–CIKs. A paired t test was used). n = 5 (for KG-1) and n = 6 (for AML#1) independent experiments using CAR-CIKs generated from different donors. (J) Cytotoxicity of CXCR4-overexpressing CD33.CAR+-CIKs after chemotaxis toward CXCL12 gradient. Migrated CIKs were harvested and cocultured for 4 hours with primary AML cells (for AML#1: ∗∗P = .002 for CD33.CAR+-CXCR4wt– vs CD33.CAR+-CIKs; ∗P = .012 for CD33.CAR+-CXCR4wt– vs CD33.CAR+-CXCR4mut–CIKs; for AML#2: ∗∗P = .002 for CD33.CAR+-CXCR4wt– vs CD33.CAR+-CIKs, ∗∗P = .009 for CD33.CAR+-CXCR4wt– vs CD33.CAR+-CXCR4mut–CIKs. A paired t test was used). For each primary AML, n = 8 independent experiments using CAR-CIKs generated from 8 different donors.