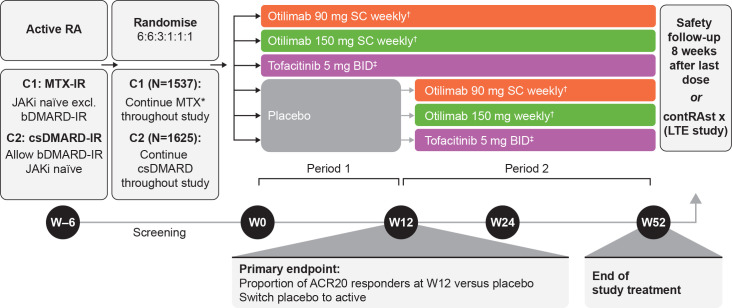
Figure 1.
ContRAst 1 and 2 study design. *Stable oral dose of MTX and ≥5 mg/week folic acid as standard of care. †Otilimab solution in vial/pre-filled syringe. ‡Tofacitinib administered as 5 mg oral capsule. ACR20, 20% improvement in American College of Rheumatology criteria; b/csDMARD, biologic/conventional synthetic disease-modifying antirheumatic drug; C1, contRAst 1; C2, contRAst 2; IR, inadequate response; JAKi, Janus kinase inhibitor; LTE, long-term extension; MTX, methotrexate; RA, rheumatoid arthritis, SC, subcutaneous; W, week.