Abstract
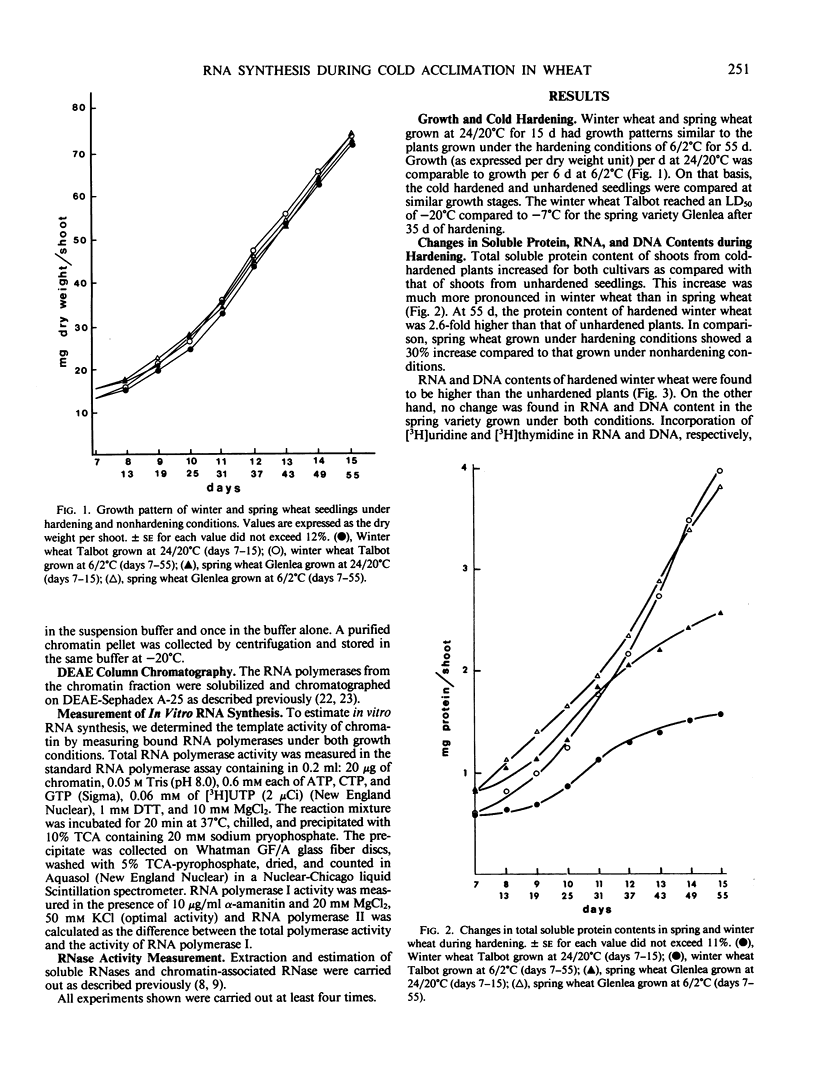
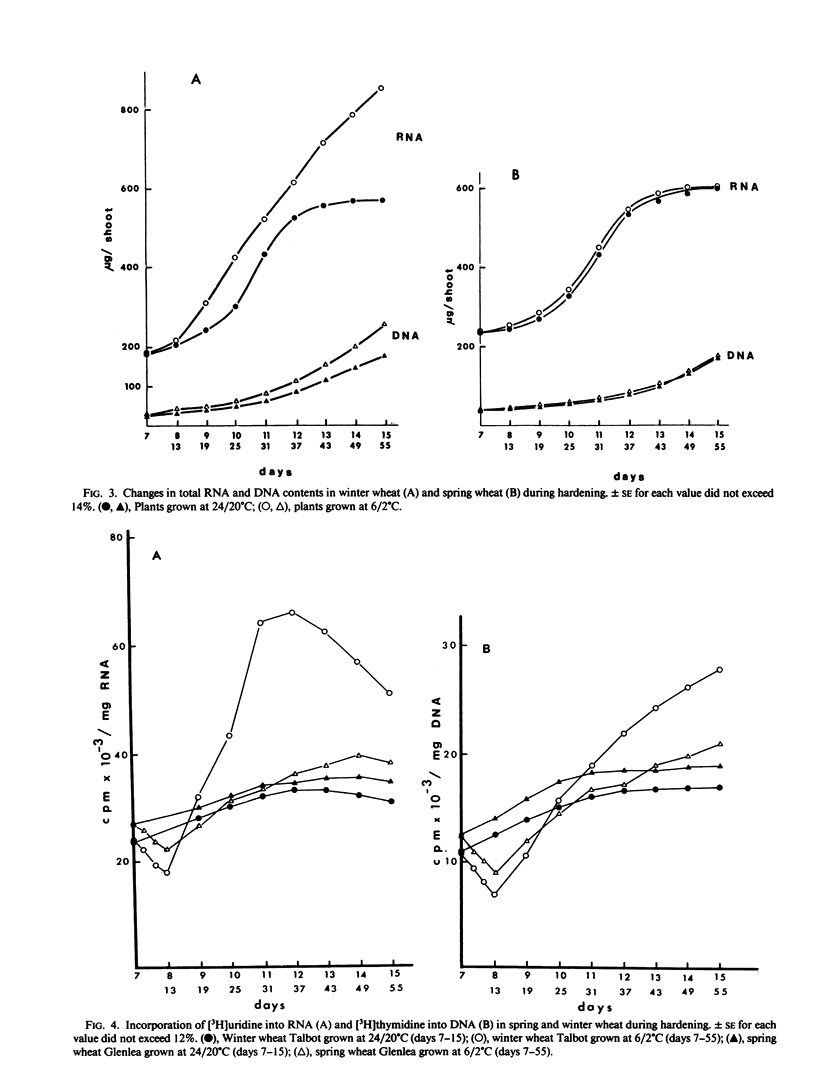
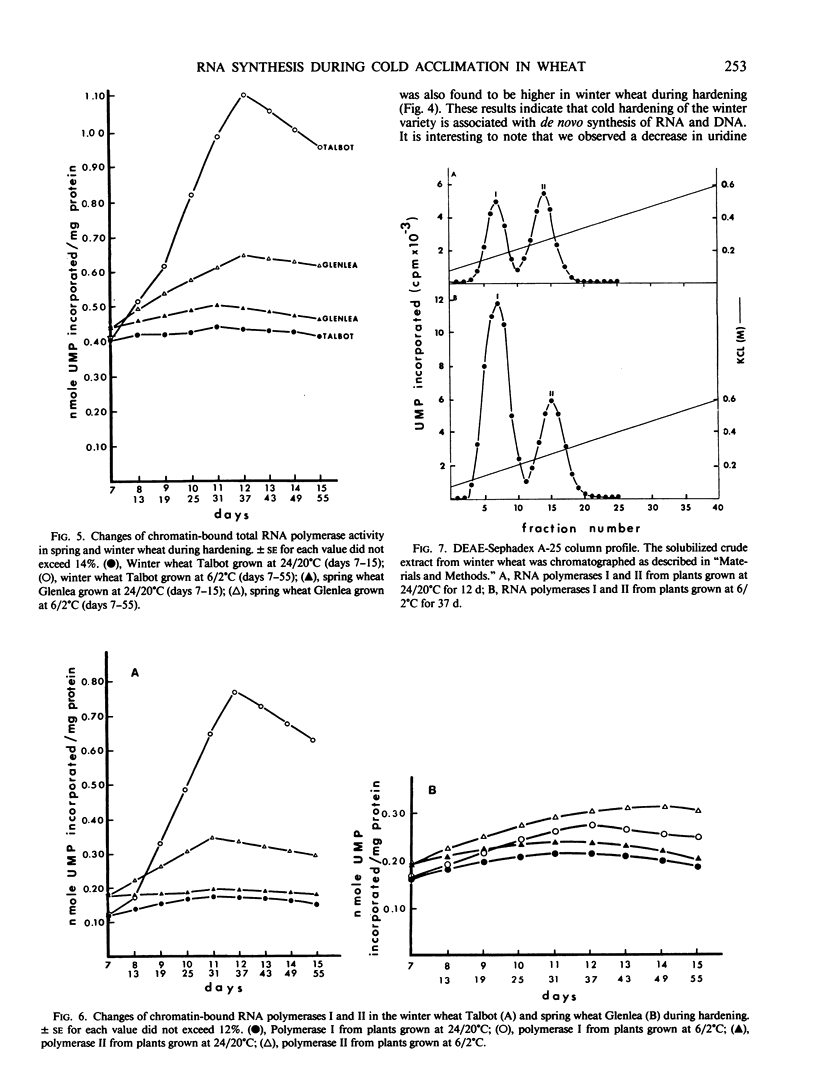
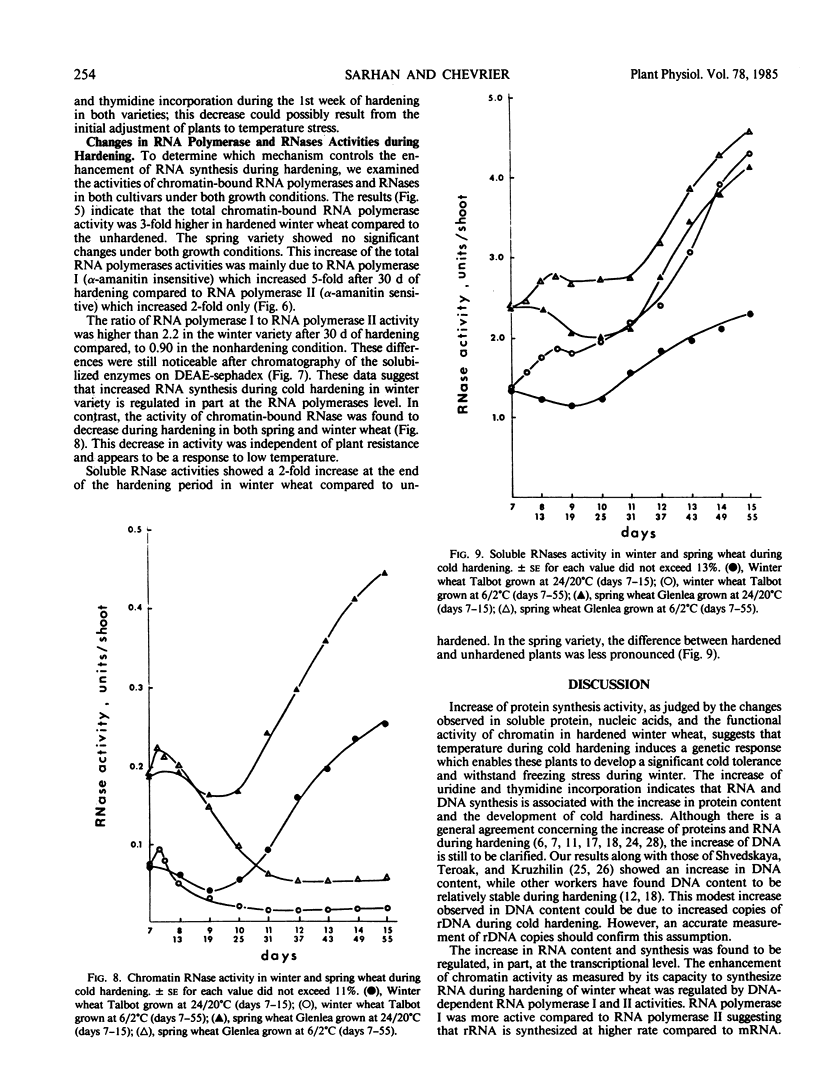
Chromatin DNA-dependent RNA polymerases and RNases activities were measured in winter and spring varieties to understand the overall regulation of RNA synthesis during cold acclimation. We found that total RNA polymerase activities were significantly higher in chromatin isolated from winter wheat compared to the spring wheat during the acclimation period. This increase was parallel to the increase in protein and RNA contents during hardening. The ratio of RNA polymerase I to RNA polymerase II activity was higher than 2 in winter wheat after 30 days of hardening compared, to a ratio of 0.90 under the nonhardening conditions. The increase in activity and the ratio of polymerase I to polymerase II was maintained after the separation of the enzymes from the template, suggesting that RNA synthesis is regulated in part at the enzyme level. On the other hand, the chromatin associated RNase activity decreased in both varieties during acclimation, indicating a nonspecific inhibition caused by low temperature rather than a selective genetic response associated with cold acclimation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. N., Bixby J. A. Ribonuclease activity during induction of cold hardiness in mimosa epicotyl and hypocotyl tissues. Cryobiology. 1973 Jun;10(2):152–156. doi: 10.1016/0011-2240(73)90022-9. [DOI] [PubMed] [Google Scholar]
- Cloutier Y. Changes in the Electrophoretic Patterns of the Soluble Proteins of Winter Wheat and Rye following Cold Acclimation and Desiccation Stress. Plant Physiol. 1983 Feb;71(2):400–403. doi: 10.1104/pp.71.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta L. V., Weiser C. J. Nucleic Acid and protein changes in relation to cold acclimation and freezing injury of korean boxwood leaves. Plant Physiol. 1972 Jan;49(1):91–96. doi: 10.1104/pp.49.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Loss of Adenosine Triphosphate Synthesis Caused by Freezing and Its Relationship to Frost Hardiness Problems. Plant Physiol. 1964 Sep;39(5):712–719. doi: 10.1104/pp.39.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury in relation to loss of enzyme activities and protection against freezing. Cryobiology. 1968 Nov-Dec;5(3):188–201. doi: 10.1016/s0011-2240(68)80163-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volger H. G., Heber U. Cryoprotective leaf proteins. Biochim Biophys Acta. 1975 Dec 15;412(2):335–349. doi: 10.1016/0005-2795(75)90048-3. [DOI] [PubMed] [Google Scholar]


