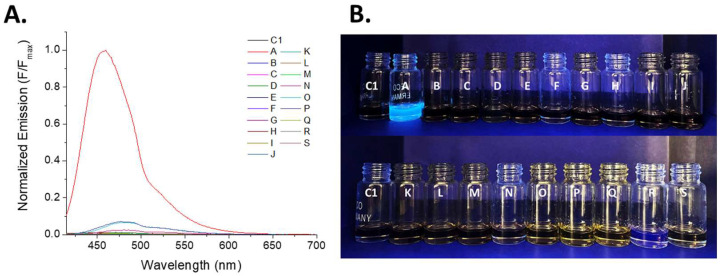
Figure 4.
Emission response of the iron (III) complex (C1) after the addition of several analytes. (A) Overlapping emission spectrum. (B) Naked-eye emission change in the complex with all analytes assessed illuminated with a UV-Lamp λ = 360 nm. CC1 = 20 µM, CSample = 10 mol equivalents, solvent = DMSO. Sample code: A. Hydroxide (OH−); B. Metabisulfite (S2O5−2); C. Iodide (I−); D. Acetate (MeCO2−); E. Nitrate (NO3−); F. Sulfate (SO4−2); G. Thiosulfate (S2O3−2); H. Bicarbonate (HCO3−); I. Bisulfite (HSO3−); J. Iodate (IO3−); K. Cyanide (CN−); L. Fluoride (F−); M. Sulfide (S−); N. Carbonate (CO3−2); O. Citrate (C6H5O7−3); P. Monoacid phosphate (HPO4−2); Q. Diacid phosphate (H2PO4−); R. Arsenite (AsO2−); S. Tartrate (C4H4O6−2).