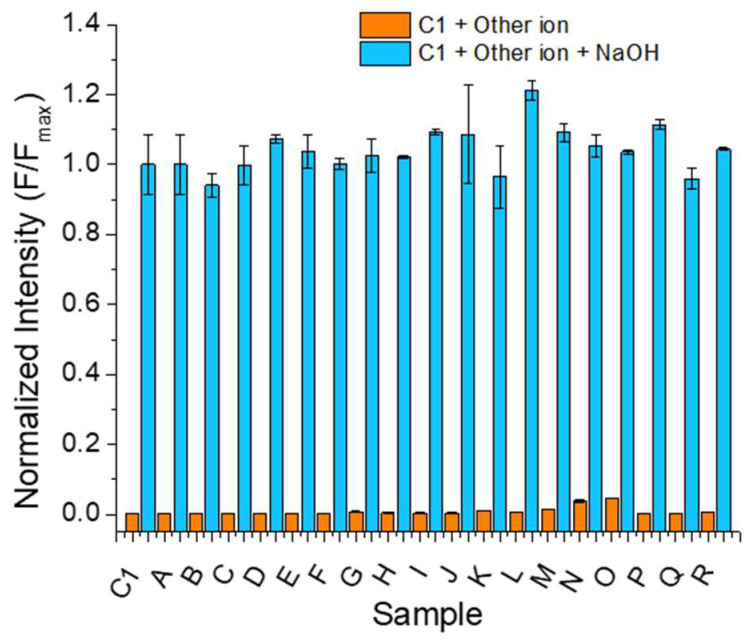
Figure 5.
Capability of NaOH to displace the interaction of the iron (III) complex (C1) with other inorganic salts. Sample coding: A. Metabisulfite (S2O5−2), B. Iodide (I−), C. Acetate (MeCO2−), D. Nitrate (NO3−), E. Sulfate (SO4−2), F. Thiosulfate (S2O3−2), G. Bicarbonate (HCO3−), H. Bisulfite (HSO3−), I. Iodate (IO3−), J. Cyanide (CN−), K. Fluoride (F−), L. Sulfide (S−2), M. Carbonate (CO3−2), N. Citrate (C6H5O7−3), O. Monoacid phosphate (HPO4−2), P. Diacid phosphate (H2PO4−), Q. Arsenite (AsO2−), R. Tartrate (C4H4O6−2). λEx = 399 nm and λEm = 456 nm. CC1= 20 µM, CSample = 10 mol equivalents, solvent = DMSO. Inorganic salts = 10 molar equivalents, incubation time = 1 min, n = 3.