Abstract
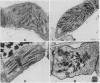
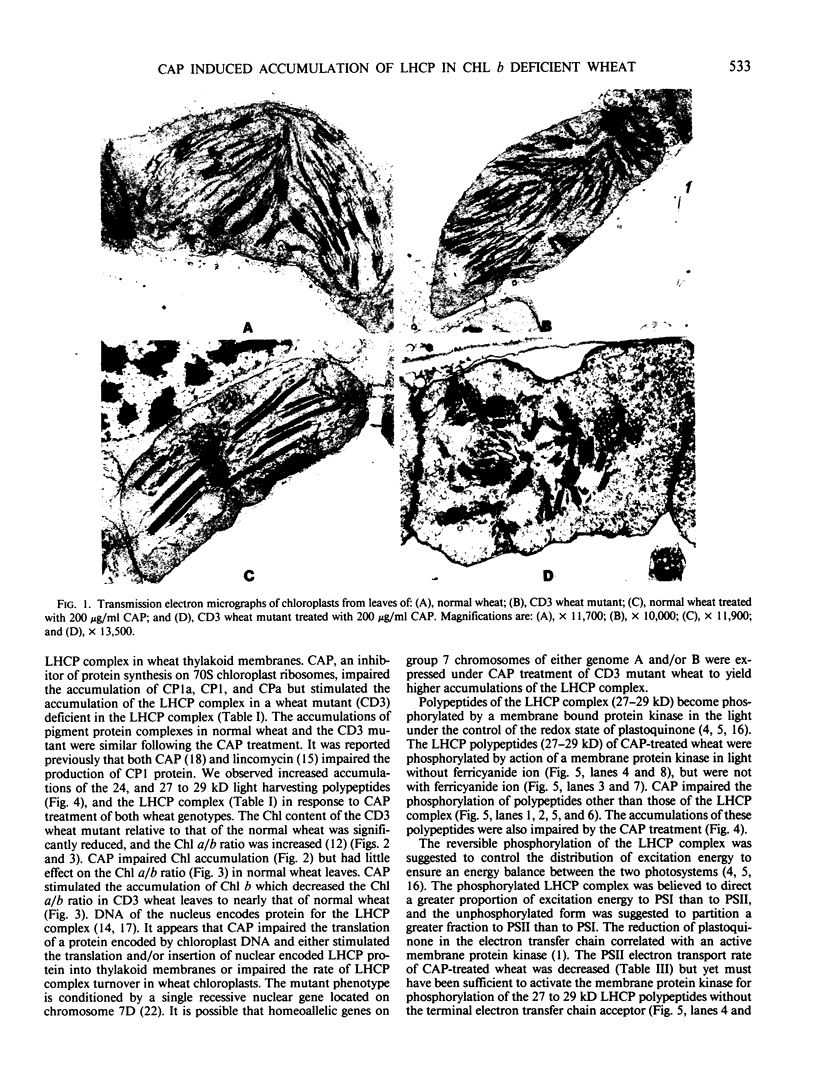
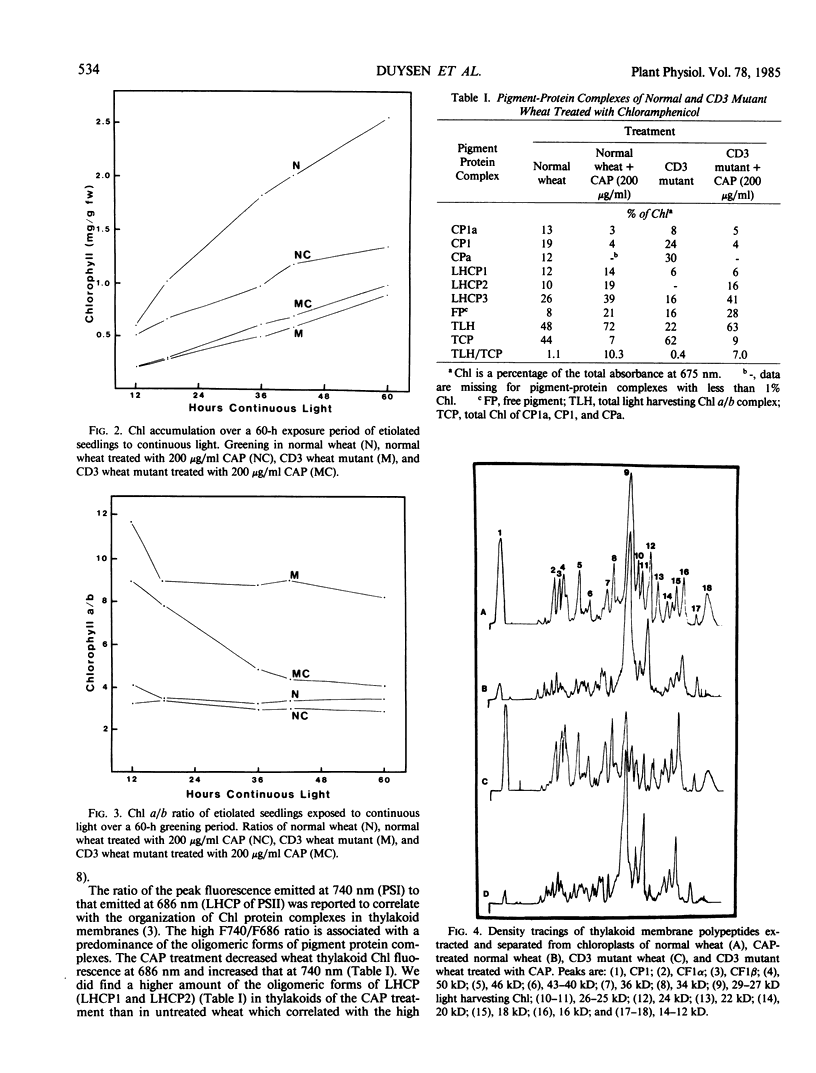
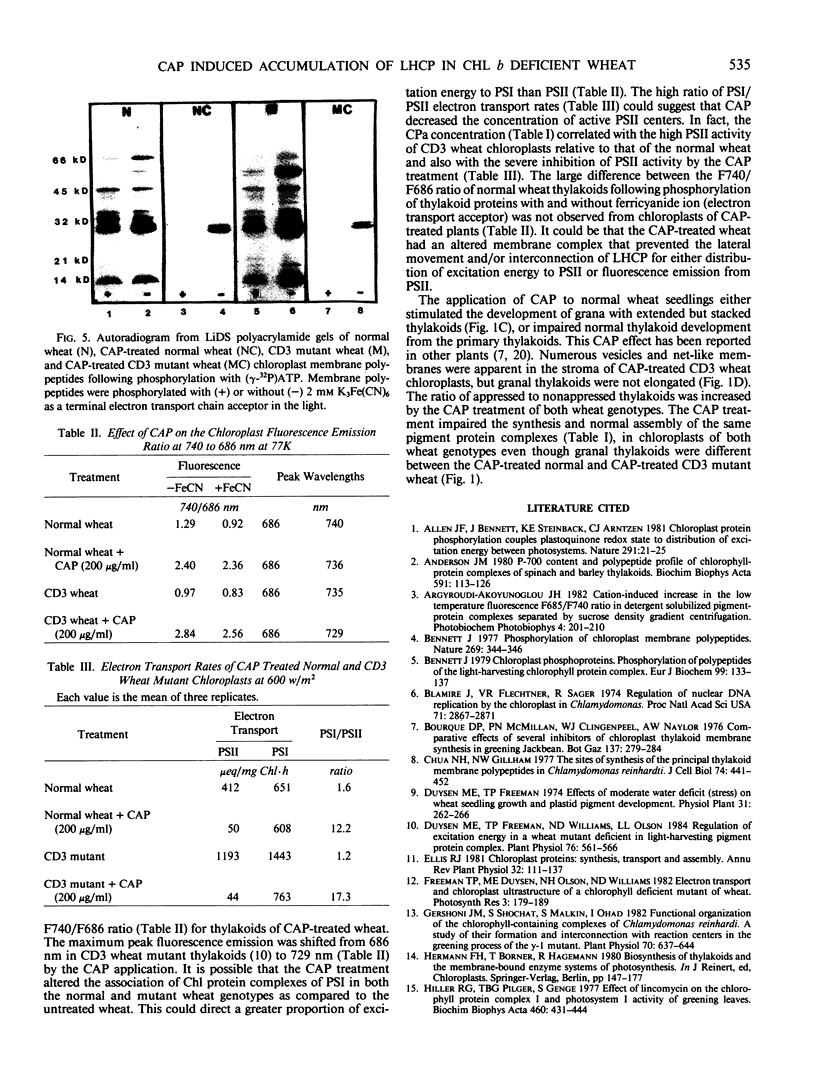
As compared with normal wheat leaves, the chlorina wheat mutant, designated CD3, has a high chlorophyll a/b ratio and a deficiency in the light harvesting chlorophyll protein (LHCP) complex. Applications of 200 micrograms per milliliter of d-threo-chloramphenicol to etiolated seedlings decreased the chlorophyll a/b ratio and increased the accumulation of the 27 kilodalton LHCP polypeptide and the LHCP complex in thylakoids of the mutant during greening. These data led to the suggestion that a protein encoded in chloroplast genes impaired either transcriptional, translational, or posttranslational events in CD3 wheat limiting the accumulation of the LHCP complex. The LHCP complex which accumulated in chloramphenicol treated wheat appeared functional even though chlorophyll protein complex accumulations were altered greatly in the wheat thylakoids. LHCP polypeptides were phosphorylated by action of a membrane protein kinase but yet photosystem II electron transport was impaired. The chloramphenicol treatment increased the photosystem I/photosystem II ratio of electron transport and the fluorescence emission ratio at 740 to 686 nanometers relative to those of untreated wheat. Chloramphenicol prevented development of normal granal thylakoids in normal wheat chloroplasts but not in those of the CD3 mutant. Elongated stacked thylakoids were observed in normal wheat. Net-like membranes and vesicles were noted in the stroma of chloroplasts from treated mutant seedlings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. P-700 content and polypeptide profile of chlorophyll-protein complexes of spinach and barley thylakoids. Biochim Biophys Acta. 1980 Jun 10;591(1):113–126. doi: 10.1016/0005-2728(80)90225-x. [DOI] [PubMed] [Google Scholar]
- Blamire J., Flechtner V. R., Sager R. Regulation of nuclear DNA replication by thechloroplast in Chlamydomonas. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2867–2871. doi: 10.1073/pnas.71.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen M. E., Freeman T. P., Williams N. D., Olson L. L. Regulation of excitation energy in a wheat mutant deficient in light-harvesting pigment protein complex. Plant Physiol. 1984 Nov;76(3):561–566. doi: 10.1104/pp.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Shochat S., Malkin S., Ohad I. Functional Organization of the Chlorophyll-Containing Complexes of Chlamydomonas reinhardi: A Study of Their Formation and Interconnection with Reaction Centers in the Greening Process of the y-1 Mutant. Plant Physiol. 1982 Sep;70(3):637–644. doi: 10.1104/pp.70.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F. H., Börner T., Hagemann R. Biosynthesis of thylakoids and the membrane-bound enzyme systems of photosynthesis. Results Probl Cell Differ. 1980;10:147–177. doi: 10.1007/978-3-540-38255-3_5. [DOI] [PubMed] [Google Scholar]
- Hiller R. G., Pilger T. B., Genge S. Effect of linocmycin on the chlorophyll protein complex I content and photosystem I activity of greening leaves. Biochim Biophys Acta. 1977 Jun 9;460(3):431–444. doi: 10.1016/0005-2728(77)90082-2. [DOI] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]