Abstract
Dietary guidance promotes plant-based foods, yet minimal research has examined intake in children. This study examined plant-based food intake in preschool-aged children using plant-based dietary index (PDI) metrics and related these metrics to nutrient and food group intakes. Dietary data were collected from preschool-aged children (n = 283, 3.45 ± 1.22 years) from the Guelph Family Health Study at baseline using the Automated Self-Administered 24-Hour Dietary Assessment Tool. Food intake servings were assigned to 16 food groups for calculation of overall PDI (oPDI), healthful PDI (hPDI), and less healthful (lhPDI) scores and summarized into tertiles for energy-adjusted comparisons. For oPDI, participants in the highest vs. lowest tertile had higher intakes of nutrients and food groups to encourage (e.g., dietary fiber, fruits) as well as lower intakes of nutrients to encourage (e.g., calcium, vitamin D). For hPDI, participants in the highest vs. lowest tertile had higher intakes of nutrients and food groups to encourage and lower intakes of those to limit (e.g., saturated fat, sweets and desserts). For lhPDI, participants in the highest vs. lowest tertile had higher intakes of nutrients and food groups to limit and lower intakes of those to encourage. These results can inform dietetic practice for dietary guidance that promotes plant-based foods in children.
Keywords: dietary guidance, dietary assessment, plant-based dietary index, nutrient intakes, food group intakes, preschool-aged children
1. Introduction
Dietary guidelines from numerous countries have shifted towards the promotion of sustainable dietary patterns that include higher intakes of plant-based foods [1,2,3,4,5]. Higher intakes of plant-based foods, including nuts, seeds, and legumes, fruits, vegetables, and whole grains, have been related to improved nutrient intake, including higher dietary fiber, micronutrients, and unsaturated fat [6], as well as improved diet quality [7]. Higher intakes of plant-based foods have also been related to improved health outcomes, including lower risk of cardiovascular disease [8,9,10,11], type 2 diabetes [12,13], breast cancer [14,15,16], and all-cause mortality [17,18,19,20]. These improvements support the dietary guidance and rationalize the measurement of plant-based food intake in various population segments.
Plant-based food intake can be measured using the plant-based dietary index (PDI), as first described by Satija et al. [12]. The PDI is designed to examine the dietary intake distribution of plant- and animal-based foods using a system that assigns positive or reverse scores to plant foods and reverse scores to animal foods [12]. The metrics include an overall PDI as well as a healthful PDI and an unhealthful PDI that reflect intake of plant-based foods that are healthy (e.g., fruits, whole grains) or less healthy (e.g., sweets and desserts, sugar sweetened beverages (SSBs)) [12]. The PDI metrics have been examined in relation to chronic disease risk in adults in various locations including North America [8,12,21,22], Europe [23,24], and Korea [10,18,25]. There is rationale to also examine PDI metrics in children, particularly since dietary habits established in young childhood can be associated with health outcomes [26] and persist into adulthood [27]. Therefore, the purpose of the current study was to examine intake of plant-based foods using the PDI metrics and relate them to nutrient and food group intakes in preschool-aged children participating in the Guelph Family Health Study (GFHS).
2. Materials and Methods
2.1. Study Design and Participant Screening
The current study used baseline dietary assessment data from the GFHS, an ongoing cohort study examining the effects of home-based lifestyle interventions on obesity prevention in families with young children. The study was approved by the University of Guelph Research Ethics Board (REB#17-07-003) and registered on ClinicalTrials.gov (NCT02939261). All parents provided written consent and, when possible, children provided verbal assent.
Participants included children between 1.5 and 5 years who were in GFHS families. Families were eligible if they resided in Guelph, Ontario, or surrounding areas, had a parent who could respond to questionnaires in English, and did not have a participating child(ren) with a severe health condition.
Of the 293 children who met the inclusionary criteria, 10 were excluded due to a missing dietary assessment (n = 1) or errors in their dietary assessment entries (n = 6), including an implausible energy intake (<500 kcal/day) (n = 3).
2.2. Anthropometric Measurements
Height was measured using a stadiometer (ShorrBoard, Weight and Measure, LLC., Olney, MD, USA). Body weight was measured using an electronic scale (BOD POD™, COSMED, Concord, CA, USA). Body mass index (BMI) z-scores were calculated using World Health Organization Anthro software (version 3.2.2, Geneva, Switzerland, 2011).
2.3. Dietary Assessment
Dietary assessment was completed by the participant’s parent for a 24 h period using the National Cancer Institute’s web-based Automated Self-Administered 24 h (ASA24) Dietary Assessment Tool, version ASA24-Canada-2016, adapted to reflect the Canadian food supply, portion sizes, and nutrient composition. ASA24 includes multiple prompts for participants to facilitate accurate data entry and has been validated for use in children [28]. ASA24-Canada analyzes the dietary data using the Canadian Nutrient File and a Health Canada recipe database along with the United States Food and Nutrient Database for Dietary Studies (FNDDS) and the Food Patterns Equivalents Database (FPED). These databases enable ASA24-Canada to output a summary of the food descriptions, energy and nutrient intakes, and United States Department of Agriculture (USDA) Food Pattern components.
2.4. Plant-Based Dietary Index (PDI) Scoring
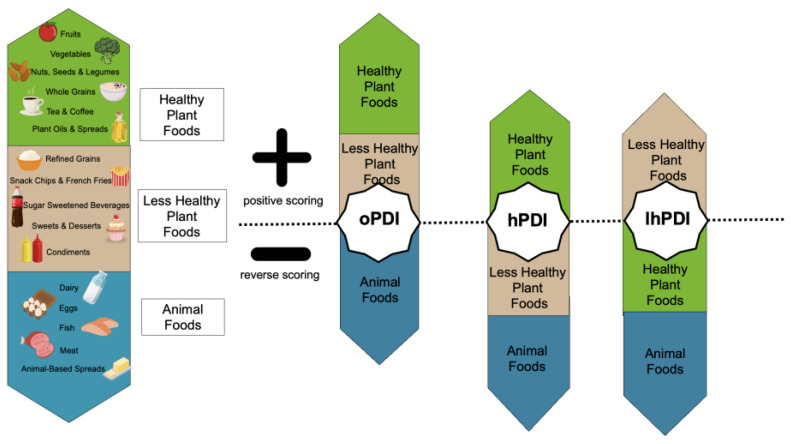
PDI scores were computed for overall PDI (oPDI), healthful PDI (hPDI), and less healthful PDI (lhPDI), adapted from Satija et al., 2016 [12]. Food intakes from the ASA24 results output were converted from grams to servings using Health Canada’s Table of Reference Amounts for Food [29]. Food descriptions reported as mixed dishes were disaggregated and quantified using information from the detailed ASA24 responses and the ASA24 food group variables, followed by conversion to grams using data from the FPED. Food servings were then assigned to 1 of 16 food groups categorized as healthy plant foods (whole grains; fruits; vegetables; nuts, seeds, and legumes; tea and coffee; plant oils and spreads), less healthy plant foods (refined grains; snack chips and French fries; SSB; sweets and desserts; condiments), or animal foods (dairy; eggs; fish; meat; animal-based spreads) (Figure 1). Servings in each food group were totalled for each participant and summarized into food group serving intake quintiles (Q) across all participants. PDI scores were then computed for each participant by relating their total intake of food group servings to the food group serving intake Qs. For oPDI, scores of 1 were assigned to all plant food group serving intakes that were in Q1 and scores of 5 were assigned to all plant food group serving intakes that were in Q5 (positive scoring such that Q1 = 1, Q2 = 2, Q3 = 3, Q4 = 4, Q5 = 5), and the opposite approach was completed for animal food group serving intakes (reverse scoring such that Q1 = 5, Q2 = 4, Q3 = 3, Q4 = 2, Q5 = 1) (Figure 1). For hPDI, positive scoring was completed for healthy plant food group serving intakes and reverse scoring was completed for less healthy plant food and animal food group serving intakes (Figure 1). For lhPDI, positive scoring was completed for less healthy plant food group serving intakes and reverse scoring was completed for healthy plant food and animal food group serving intakes (Figure 1). Scores were summed within a participant for oPDI, hPDI, and lhPDI with theoretical ranges of 16 to 80.
Figure 1.
Summary of the PDI metrics scoring process. Abbreviations used: oPDI, overall plant-based dietary index; hPDI, healthful plant-based dietary index; lhPDI, less healthful plant-based dietary index. Created with Canva, adapted with permission from Sarah E. Jarvis.
2.5. Data and Statistical Analysis
All data were analyzed using the Statistical Analysis System (SAS Institute Inc., Version 9.4, Cary, NC, USA) with p < 0.05 considered significant. All dietary data were examined for normality using box plots and stem-leaf diagrams and log-transformed where appropriate. Summary statistics were generated for sex, BMI z-score, and PDI scores, and tertiles were computed for PDI scores. Nutrient and food group intakes were compared among oPDI, hPDI, and lhPDI tertiles using the GENMOD procedure (to implement the generalized estimating equation approach to control for correlated outcomes among siblings), adjusted for energy intake, and followed by a Tukey’s test for multiple comparisons.
3. Results
3.1. Participant Characteristics
Participants included 148 girls and 135 boys who had a mean ± SD age of 3.45 ± 1.22 years and BMI z-score of 0.58 ± 0.98.
3.2. oPDI Scores in Relation to Nutrient and Food Group Intakes
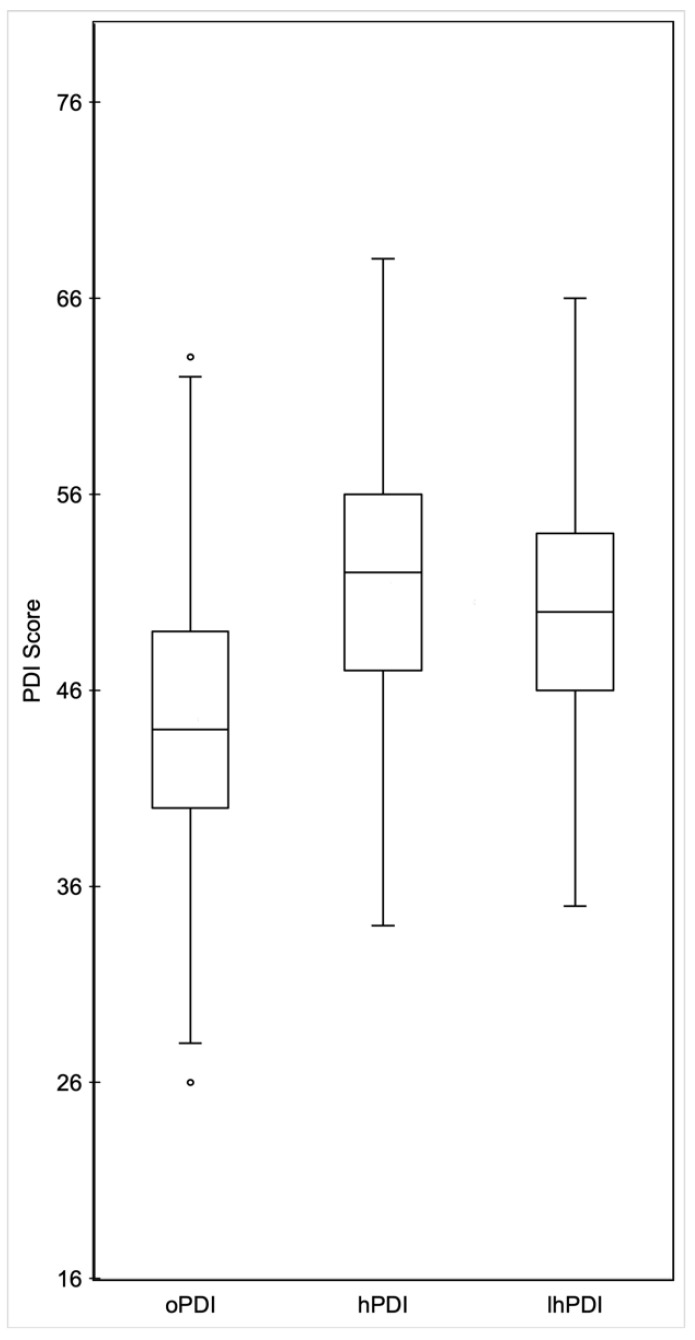
The median oPDI score was 42 with a range of 26–63 (Figure 2). Tertiles for oPDI were 26–41 (n = 105) for tertile 1, 42–47 (n = 87) for tertile 2, and 48–63 (n = 91) for tertile 3.
Figure 2.
PDI metric box plots. Distribution of PDI scores of children for oPDI, hPDI, and lhPDI. Abbreviations: PDI, plant-based dietary index; oPDI, overall plant-based dietary index; hPDI, healthful plant-based dietary index; lhPDI, less healthful plant-based dietary index.
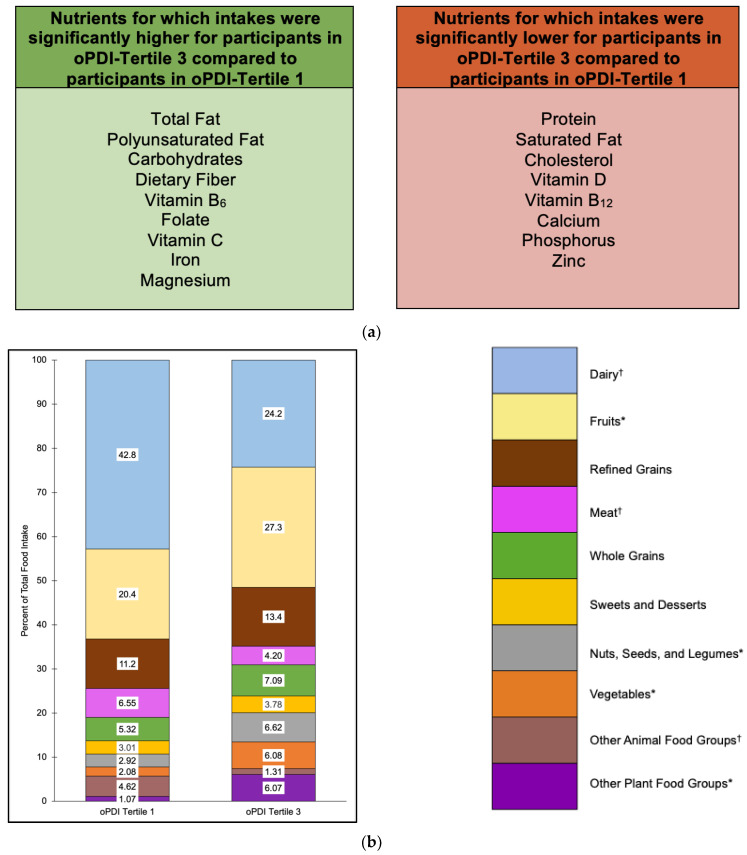
Nutrient intakes that were significantly higher for participants in oPDI tertile 3 compared to tertile 1 included total fat (p = 0.0004), polyunsaturated fat (p = 0.03), carbohydrates (p < 0.0001), dietary fiber (p < 0.0001), vitamin B6 (p = 0.03), folate (p = 0.005), vitamin C (p = 0.0002), iron (p < 0.0001), and magnesium (p < 0.0001) (Figure 3a and Table S1, Supplementary Data). Nutrient intakes that were significantly lower for participants in oPDI tertile 3 compared to tertile 1 included protein (p < 0.0001), saturated fat (p < 0.0001), cholesterol (p < 0.0001), vitamin D (p < 0.0001), vitamin B12 (p < 0.0001), calcium (p < 0.0001), phosphorus (p < 0.0001), and zinc (p = 0.005) (Figure 3a and Table S1, Supplementary Data).
Figure 3.
Nutrient intakes (a) and food group proportional intakes (b) of participants in oPDI tertile 3 compared to oPDI tertile 1. Values within each food group bar segment are percent of total food intake. Other animal food groups and other plant food groups are combinations of food groups with intakes ≤3% of total food intake for both tertiles 1 and 3. Intakes for food groups in the legend with * were significantly higher for oPDI tertile 3 compared to oPDI tertile 1 (within the other plant food groups, this refers to snack chips and French fries, sugar-sweetened beverages, and condiments). Intakes for food groups in the legend with † were significantly lower for oPDI tertile 3 compared to oPDI tertile 1 (within the other animal food groups, this refers to eggs and animal-based spreads). Abbreviations: oPDI, overall plant-based dietary index.
Food groups that contributed the highest proportions of food intake for oPDI tertiles 1 and 3 included dairy (42.8% and 24.2%, respectively), fruits (20.4% and 27.3%, respectively), and refined grains (11.2% and 13.4%, respectively) (Figure 3b). Food groups that contributed the lowest proportions of food intake accounted for ≤3% of total food intake for oPDI tertiles 1 and 3 and included SSB (0% and 2.42%, respectively), snack chips and French fries (0.34% and 1.30%, respectively), condiments (0.30% and 1.49%, respectively), plant oils and spreads (0.22% and 0.43%, respectively), tea and coffee (0.21% and 0.43%, respectively), eggs (2.97% and 0.92%, respectively), animal-based spreads (0.91% and 0.28%, respectively), and fish (0.74% and 0.11%, respectively) (Figure 3b).
Food group proportional intakes that were significantly higher for participants in oPDI tertile 3 compared to tertile 1 included fruits (p = 0.003); nuts, seeds, and legumes (p = 0.01); vegetables (p = 0.002); snack chips and French fries (p = 0.02), SSB (p = 0.03); and condiments (p < 0.0001) (Figure 3b). Food group proportional intakes that were significantly lower for participants in oPDI tertile 3 compared to tertile 1 included dairy (p < 0.0001), meat (p = 0.02), eggs (p = 0.0002), and animal-based spreads (p < 0.0001) (Figure 3b).
3.3. hPDI Scores in Relation to Nutrient and Food Group Intakes
The median hPDI score was 52 with a range of 34–68 (Figure 2). Tertiles for hPDI were 34–49 (n = 98) for tertile 1, 50–55 (n = 97) for tertile 2 and 56–68 (n = 88) for tertile 3.
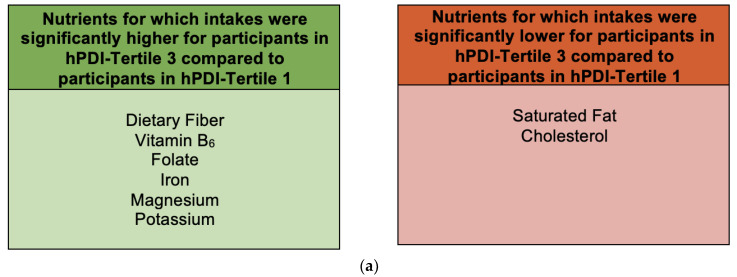
Nutrient intakes that were significantly higher for participants in hPDI tertile 3 compared to tertile 1 included dietary fiber (p < 0.0001), vitamin B6 (p = 0.009), folate (p = 0.004), iron (p = 0.004), magnesium (p < 0.0001), and potassium (p < 0.0001) (Figure 4a and Table S2, Supplementary Data). Nutrient intakes that were significantly lower for participants in hPDI tertile 3 compared to tertile 1 included saturated fat (p = 0.003) and cholesterol (p = 0.0002) (Figure 4a and Table S2, Supplementary Data).
Figure 4.
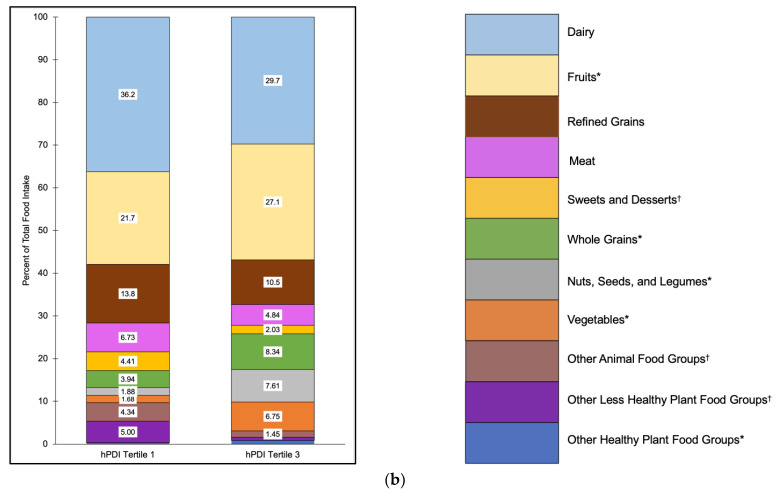
Nutrient intakes (a) and food group proportional intakes (b) of participants in hPDI tertile 3 compared to hPDI tertile 1. Values within each food group bar segment are percent of total food intake. Other animal food groups, other less healthy plant food groups, and other healthy plant food groups are combinations of food groups with intakes ≤3% of total food intake for both tertiles 1 and 3. Intakes for food groups in the legend with * were significantly higher for hPDI tertile 3 compared to tertile 1 (within the other healthy plant food groups, this refers to plant oils and spreads). Intakes for food groups in the legend with † were significantly lower for hPDI tertile 3 compared to hPDI tertile 1 (within the other animal food groups, this refers to eggs and animal-based spreads, and within the other less healthy plant food groups, this refers to snack chips and French fries, sugar-sweetened beverages, and condiments). Abbreviations: hPDI, healthful plant-based dietary index.
Food groups that contributed the highest proportions of food intake for hPDI tertiles 1 and 3 included dairy (36.2% and 29.7%, respectively), fruits (21.7% and 27.1%, respectively), and refined grains (13.8% and 10.5%, respectively) (Figure 4b). Food groups that contributed the lowest proportions of food intake accounted for ≤3% of total food intake for hPDI tertiles 1 and 3 and included SSB (2.05% and 0.16%, respectively), snack chips and French fries (1.70% and 0.12%, respectively), condiments (1.25% and 0.49%, respectively), tea and coffee (0.26% and 0.48%, respectively), plant oils and spreads (0.08% and 0.39%, respectively), eggs (2.47% and 0.94%, respectively), animal-based spreads (0.96% and 0.29%, respectively), and fish (0.91% and 0.22%, respectively) (Figure 4b).
Food group proportional intakes that were significantly higher for participants in hPDI tertile 3 compared to tertile 1 included fruits (p = 0.03), whole grains (p = 0.0004), nuts, seeds, and legumes (p < 0.0001), vegetables (p = 0.0005), and plant oils and spreads (p < 0.0001) (Figure 4b). Food group proportional intakes that were significantly lower for participants in hPDI tertile 3 compared to tertile 1 included sweets and desserts (p = 0.002), eggs (p = 0.008), animal-based spreads (p < 0.0001), snack chips and French fries (p < 0.0001), SSB (p = 0.03), and condiments (p = 0.02) (Figure 4b).
3.4. lhPDI Scores in Relation to Nutrient and Food Group Intakes
The median lhPDI score was 50 with a range of 35–66 (Figure 2). Tertiles for oPDI were 35–47 (n = 101) for tertile 1, 48–52 (n = 86) for tertile 2, and 53–66 (n = 96) for tertile 3.
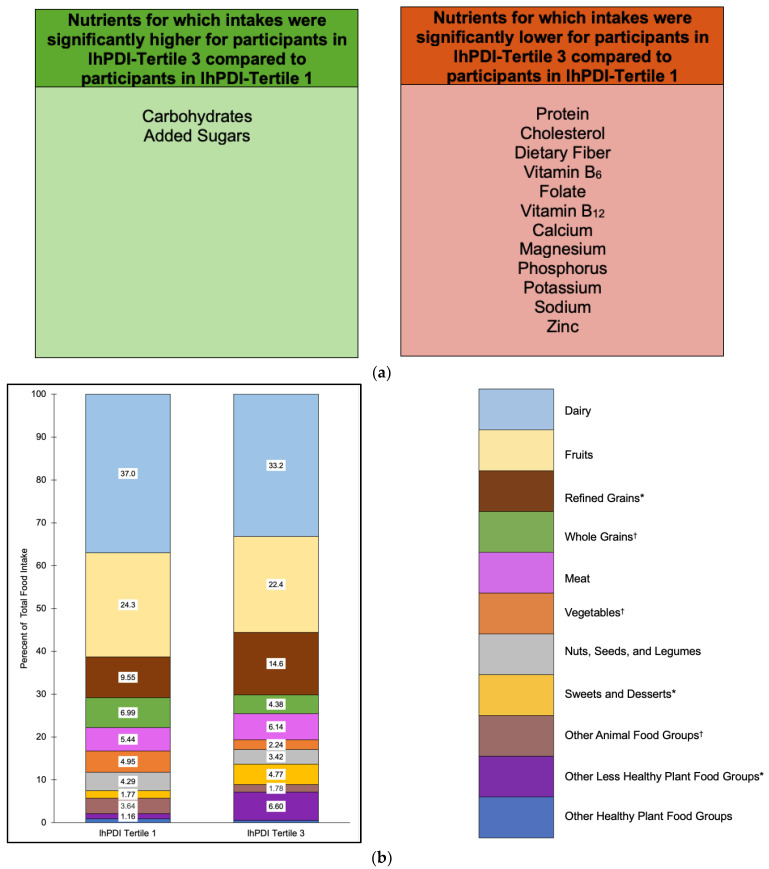
Nutrient intakes that were significantly higher for participants in lhPDI tertile 3 compared to tertile 1 included carbohydrates (p = 0.001) and added sugars (p < 0.0001) (Figure 5a and Table S2, Supplementary Data). Nutrient intakes that were significantly lower for participants in lhPDI tertile 3 compared to tertile 1 included protein (p < 0.0001), cholesterol (p < 0.0001), dietary fiber (p < 0.0001), vitamin B6 (p = 0.002), folate (p = 0.01), vitamin B12 (p = 0.003), calcium (p = 0.0005), magnesium (p < 0.0001), phosphorus (p < 0.0001), potassium (p < 0.0001), sodium (p = 0.04), and zinc (p < 0.0001) (Figure 5a and Table S2, Supplementary Data).
Figure 5.
Nutrient intakes (a) and food group proportional intakes (b) of participants in lhPDI tertile 3 compared to lhPDI tertile 1. Values within each food group bar segment are percents of total food intake. Other animal food groups, other less healthy plant food groups, and other healthy plant food groups are combinations of food groups with intakes ≤3% for both tertiles 1 and 3. Intakes for food groups in the legend with * were significantly higher for lhPDI tertile 3 compared to lhPDI tertile 1 (within the other less healthy plant food groups, this refers to snack chips and French fries, and condiments). Intakes for food groups in the legend with † were significantly lower for lhPDI tertile 3 compared to lhPDI tertile 1 (within the other animal food groups, this refers to fish). Abbreviations: lhPDI, less healthful plant-based dietary index.
Food groups that contributed the highest proportions of food intake for lhPDI tertiles 1 and 3 included dairy (37.0% and 33.2%, respectively), fruits (24.3% and 22.4%, respectively), and refined grains (9.55% and 14.6%, respectively) (Figure 5b). Food groups that contributed the lowest proportions of food intake accounted for ≤3% of total food intake for lhPDI tertiles 1 and 3 and included SSB (0.69% and 3.02%, respectively), snack chips and French fries (0.13% and 2.17%, respectively), condiments (0.34% and 1.41%, respectively), tea and coffee (0.59% and 0.25%, respectively), plant oils and spreads (0.34% and 0.27%, respectively), eggs (2.32% and 1.23%, respectively), animal-based spreads (0.63% and 0.49%, respectively), and fish (0.69% and 0.06%, respectively) (Figure 5b).
Food group proportional intakes that were significantly higher for participants in lhPDI tertile 3 compared to tertile 1 included refined grains (p = 0.0009), sweets and desserts (p < 0.0001), snack chips and French fries (p < 0.0001), and condiments (p = 0.0003) (Figure 5b). Food group proportional intakes that were significantly lower for participants in lhPDI tertile 3 compared to tertile 1 included whole grains (p = 0.02), vegetables (p = 0.03), and fish (p = 0.008) (Figure 5b).
4. Discussion
The current study examined plant-based food intake in a sample of 283 preschool-aged children who were participating in the GFHS. Dietary assessment was completed for a 24 h period by each child’s parent using the online-based ASA24. The itemized food intakes were converted to servings and categorized into 11 plant food groups or 5 animal food groups for calculation of oPDI scores. Plant food groups were further categorized into healthy or less healthy plant food groups for calculation of hPDI and lhPDI scores. The focus on dietary distribution of plant-based food intakes in young children is relevant as it is a critical stage for growth and development, with unique nutritional requirements [30]. Gaining insights into the nutritional implications of plant-based foods in children’s diets is pertinent as evidence demonstrates that childhood dietary habits can persist into adulthood [27]. As such, the current study examined the PDI metric scores and related them to nutrient and food group intakes in a sample of preschool-aged children.
The current study’s focus on young children in its examination of plant-based food intake adds diversity to the participants that have been studied in this literature. Adults have been the focus of most of the previous studies of plant-based food intake, which have related PDI metrics to various health conditions [12,14,19,25]. The need for research conducting thorough examinations of plant-based food intake in children is important since childhood dietary habits can continue through into adulthood [27] and relate to health outcomes [26]. Overall, since dietary guidance includes children, the current study’s participant sample adds necessary diversity to the plant-based food intake literature.
The range of oPDI scores in the current study is 26–63 out of a theoretical range of 16–80. When oPDI scores are summarized into tertiles, the results show that participants who have higher overall plant intake (oPDI-tertile 3) have higher intakes of nutrients to encourage (polyunsaturated fat, dietary fiber, vitamin B6, folate, vitamin C, iron, magnesium), and, except for saturated fat and cholesterol, also have lower intakes of nutrients to encourage (protein, vitamin D, vitamin B12, calcium, phosphorus, zinc). The food group intakes accounted for in these nutrient intakes show that participants who have higher oPDI have higher intakes of fruits, vegetables, nuts, seeds, and legumes, and lower intakes of dairy and meat. The majority of PDI studies have been conducted in adults and focus on health outcomes; however, Chen et al. studied children aged 6–9 years living in China [31]. Their food group results show that participants with higher overall plant intake have higher intakes of healthy plant foods and also some less healthy plant foods, although statistical comparisons are not completed and nutrient intakes are not reported [31]. Other PDI studies that have examined nutrient intakes report higher PDI scores in relation to higher intakes of carbohydrates [32,33], polyunsaturated fat [33], dietary fiber and vitamin B6 [32,33], folate [32], vitamin C [33,34], and magnesium [32,33], and lower intakes of protein [15,21,33,34], total fat [15,33,34], saturated fat [15,33,34], cholesterol, vitamin B6, vitamin B12, calcium, and magnesium [15,33], although all of these studies were conducted in Iranian adults, except for one that was in South Korean adults [21]. Collectively, these studies support a role for plant foods in promoting the intake of nutrients beneficial for health, but also demonstrate that other nutrients beneficial for health can be lower with varying intakes of certain plant foods. These results argue for a comprehensive dietary approach that includes a diversity of plant foods to support optimal nutrient intake.
The hPDI metric further examines plant food intake by considering the intake of plant foods that are considered healthy. The range of hPDI scores in the current study is 34–68 out of a theoretical range of 16–80. Participants who have higher intakes of healthy plant foods (hPDI-tertile 3) have higher intakes of nutrients to encourage (dietary fiber, vitamin B6, folate, iron, magnesium, potassium) and lower intakes of a nutrient to limit (saturated fat). These results correspond with higher food group intakes, including higher intakes of healthy plant foods (fruits, whole grains, nuts, seeds, and legumes, vegetables, and plant oils and spreads). Previous PDI studies also report higher hPDI scores in relation to higher intakes of dietary fiber [9,15,33,35], vitamin B6 [15,33], folate [9,15,21], iron [21], and magnesium [9,15,21], and lower intakes of saturated fat [9,15,21,33,35], although all of these studies were conducted in adults. These results demonstrate that higher hPDI scores reflect a diet high in food groups to encourage and contribute to optimal nutrient intakes by promoting increased intakes of nutrients to encourage and lower intakes of nutrients to limit.
The lhPDI metric also further examines plant food intake by considering the intake of plant foods that are considered less healthy. The range of lhPDI scores is 35–66 out of a theoretical range of 16–80. Participants who have higher intakes of less healthy plant foods (lhPDI-tertile 3) have higher intakes of nutrients and food groups to limit (added sugars, sweets and desserts, and snack chips and French Fries) and lower intakes of nutrients and food groups to encourage (protein, dietary fiber, several micronutrients, whole grains, vegetables, and fish). These results are consistent with previous studies, all conducted in adults, that also report higher lhPDI scores in relation to higher intakes of added sugars [35], and lower intakes of protein [21,33], dietary fiber [15,21,33,35], and micronutrients, including vitamin B6 [15,33], folate [15,21], calcium, magnesium, and potassium [21,33]. These findings highlight the consideration of nutritional quality when relating plant food intake to nutrient intakes. A higher intake of plant foods may not always be consistent with higher intakes of nutrients and food groups to encourage, which rationalizes the inclusion of the hPDI and lhPDI metrics in the dietary assessment of plant food intake.
In this study sample of preschool-aged children, dairy, fruits, and refined grains were the most frequently consumed food groups, accounting for >60% of total food intake, regardless of PDI metric or tertile. These food group intake results are consistent with previous studies that report children aged 2–6 years in China most frequently consume cereals, dairy, and fruits [36], and children aged 2–3 years in the United States consume milk and fruit at least once daily [37]. Dairy foods are nutrient-dense, providing high-quality protein and many micronutrients, including calcium and vitamin D to support growth and development in children [38]. Fruits also provide multiple micronutrients and can be high in dietary fiber, which can all support health [39]. Refined grains can be a source of multiple shortfall micronutrients, including folic acid and iron, to contribute toward nutrient adequacy [40]. Nonetheless, dietary intake can always be improved with more variety and increased intakes of certain foods such as vegetables, and nuts, seeds, and legumes, which can contribute several nutrients to encourage, including dietary fiber, polyunsaturated fat, monounsaturated fat, and multiple micronutrients [41,42]. These findings demonstrate a need for greater diversity in children’s diets to promote nutrient intake adequacy and foster lifelong healthy dietary habits.
The current study is limited in its use of a single, self-reported 24 h dietary recall, which has inherent recall bias and/or measurement error. A strength of this study is its focus on children, who have been less studied, despite evidence that eating habits at a young age can persist into adulthood [27]. Another strength is the rigorous process employed to disaggregate mixed dishes into individual foods, which contributed to an increased accuracy of food group classification and subsequent PDI metric scoring.
5. Conclusions
In conclusion, the current study examined plant food intake in a sample of 283 preschool-aged children using PDI metrics. The results show that participants who have higher intakes of plant foods (oPDI) have higher intakes of nutrients and food groups to encourage (e.g., dietary fiber, fruits) but also lower intakes of nutrients to encourage (e.g., calcium, vitamin D). When plant food intake is further examined according to healthfulness, results predictably show that participants who have higher intakes of healthy plant foods (hPDI) have higher intakes of nutrients and food groups to encourage (e.g., dietary fiber, fruits), while participants who have higher intakes of less healthy plant foods (lhPDI) have higher intakes of nutrients and food groups to limit (e.g., added sugars and snack chips and French fries). These results provide evidence of the types of plant foods that preschool-aged children are consuming, and support directions for dietetic practice. Future research can include examinations of PDI scores in relation to health outcomes in children and effects of interventions on PDI scores in children. Overall, the results of this study can contribute toward the development of nutritional strategies that facilitate plant food intake in children.
Acknowledgments
The authors acknowledge the families who participanted in the GFHS, Angela Annis and Madeline Nixon for their work in coordinating the GFHS, and Adam Sadowski and Amar Laila for their data analytical expertise.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/2072-6643/15/21/4617/s1: Table S1: Nutrient intakes by oPDI tertiles in preschool-aged children; Table S2: Nutrient intakes by hPDI and lhPDI tertiles in preschool-aged children.
Author Contributions
A.M.D., P.F.C.A. and O.A.L. conceptualized the study; P.F.C.A., O.A.L., Z.J.R. and A.M.D. completed the data analyses; D.W.L.M. and J.H. are the Co-Directors of the GFHS and conceptualized the GFHS and supervised the GFHS data collection; A.M.D. oversaw all study activities; P.F.C.A., O.A.L. and A.M.D. wrote the manuscript; and all authors reviewed and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Board of the University of Guelph (REB#17-07-003).
Informed Consent Statement
All parents provided written consent and, when possible, children provided assent.
Data Availability Statement
Interested researchers can contact GFHS investigators to explore data availability in alignment with the University of Guelph Research Ethics Board.
Conflicts of Interest
The authors declare no conflict of interest related to this work.
Funding Statement
This research was funded by the Health for Life Initiative at the University of Guelph.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.U.S. Department of Agriculture. U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020–2025; 9th ed.; December 2020. [(accessed on 10 May 2023)]; Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf.
- 2.National Health and Medical Research Council . Australian Dietary Guidelines. National Health and Medical Research Council; Canberra, Australia: 2013. [Google Scholar]
- 3.High Council for Public Health, Paris, Île-de-France, France French Nutrition and Health Programme’s Dietary Guidelines for Adults. 2017. [(accessed on 14 May 2023)]. Available online: https://www.fao.org/nutrition/education-nutritionnelle/food-dietary-guidelines/regions/france/previous-versions/fr/
- 4.Ministry of Food, Agriculture and Fisheries of Denmark The Official Dietary Guidelines Good for Health and Climate. [(accessed on 16 May 2023)]. Available online: https://foedevarestyrelsen.dk/publikationer/2021/the-official-dietary-guidelines-good-for-health-and-climate-booklet.
- 5.Health Canada Canada’s Dietary Guidelines For Health Professionals and Policy Makers. 2019. [(accessed on 25 May 2023)]. Available online: https://food-guide.canada.ca/sites/default/files/artifact-pdf/CDG-EN-2018.pdf.
- 6.Neufingerl N., Eilander A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients. 2021;14:29. doi: 10.3390/nu14010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker H.W., Vadiveloo M.K. Diet quality of vegetarian diets compared with nonvegetarian diets: A systematic review. Nutr. Rev. 2019;77:144–160. doi: 10.1093/nutrit/nuy067. [DOI] [PubMed] [Google Scholar]
- 8.Satija A., Bhupathiraju S.N., Spiegelman D., Chiuve S.E., Manson J.E., Willett W., Rexrode K.M., Rimm E.B., Hu F.B. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J. Am. Coll. Cardiol. 2017;70:411–422. doi: 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heianza Y., Zhou T., Sun D., Hu F.B., Qi L. Healthful plant-based dietary patterns, genetic risk of obesity, and cardiovascular risk in the UK biobank study. Clin. Nutr. 2021;40:4694–4701. doi: 10.1016/j.clnu.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song S., Lee K., Park S., Shin N., Kim H., Kim J. Association between Unhealthful Plant-Based Diets and Possible Risk of Dyslipidemia. Nutrients. 2021;13:4334. doi: 10.3390/nu13124334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouvari M., Tsiampalis T., Chrysohoou C., Georgousopoulou E., Skoumas J., Mantzoros C.S., Pitsavos C.S., Panagiotakos D.B. Quality of plant-based diets in relation to 10-year cardiovascular disease risk: The ATTICA cohort study. Eur. J. Nutr. 2022;61:2639–2649. doi: 10.1007/s00394-022-02831-0. [DOI] [PubMed] [Google Scholar]
- 12.Satija A., Bhupathiraju S.N., Rimm E.B., Spiegelman D., Chiuve S.E., Borgi L., Willett W.C., Manson J.E., Sun Q., Hu F.B. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016;13:e1002039. doi: 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F., Baden M.Y., Guasch-Ferré M., Wittenbecher C., Li J., Li Y., Wan Y., Bhupathiraju S.N., Tobias D.K., Clish C.B., et al. Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes. Diabetologia. 2022;65:1119–1132. doi: 10.1007/s00125-022-05692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasanfar B., Toorang F., Booyani Z., Vassalami F., Mohebbi E., Azadbakht L., Zendehdel K. Adherence to plant-based dietary pattern and risk of breast cancer among Iranian women. Eur. J. Clin. Nutr. 2021;75:1578–1587. doi: 10.1038/s41430-021-00869-7. [DOI] [PubMed] [Google Scholar]
- 15.Rigi S., Mousavi S.M., Benisi-Kohansal S., Azadbakht L., Esmaillzadeh A. The association between plant-based dietary patterns and risk of breast cancer: A case-control study. Sci. Rep. 2021;11:3391. doi: 10.1038/s41598-021-82659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanos-Nanclares A., Willett W.C., Rosner B.A., Collins L.C., Hu F.B., Toledo E., Eliassen A.H. Healthful and unhealthful plant-based diets and risk of breast cancer in U.S. women: Results from the Nurses' Health Studies. Cancer Epidemiol. Biomark. Prev. 2021;30:1921–1931. doi: 10.1158/1055-9965.EPI-21-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-González M.A., Sánchez-Tainta A., Corella D., Salas-Salvadó J., Ros E., Arós F., Gómez-Gracia E., Fiol M., Lamuela-Raventós R.M., Schröder H., et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 2014;100:320S–3208S. doi: 10.3945/ajcn.113.071431. [DOI] [PubMed] [Google Scholar]
- 18.Kim J., Kim H., Giovannucci E.L. Plant-based diet quality and the risk of total and disease-specific mortality: A population-based prospective study. Clin. Nutr. 2021;40:5718–5725. doi: 10.1016/j.clnu.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Weston L.J., Kim H., Talegawkar S.A., Tucker K.L., Correa A., Rebholz C.M. Plant-based diets and incident cardiovascular disease and all-cause mortality in African Americans: A cohort study. PLoS Med. 2022;19:e1003863. doi: 10.1371/journal.pmed.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidemann C., Schulze M.B., Franco O.H., van Dam R.M., Mantzoros C.S., Hu F.B. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–237. doi: 10.1161/CIRCULATIONAHA.108.771881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H., Rebholz C.M., Garcia-Larsen V., Steffen L.M., Coresh J., Caulfield L.E. Operational differences in plant-based diet indices affect the ability to detect associations with incident hypertension in middle-aged US adults. J. Nutr. 2020;150:842–850. doi: 10.1093/jn/nxz275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., Drouin-Chartier J.P., Li Y., Baden M.Y., Manson J.E., Willett W.C., Voortman T., Hu F.B., Bhupathiraju S.N. Changes in plant-based diet indices and subsequent risk of type 2 diabetes in women and men: Three U.S. prospective cohorts. Diabetes Care. 2021;44:663–671. doi: 10.2337/dc20-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laouali N., Shah S., MacDonald C.J., Mahamat-Saleh Y., El Fatouhi D., Mancini F., Fagherazzi G., Boutron-Ruault M.C. BMI in the associations of plant-based diets with type 2 diabetes and hypertension risks in women: The E3N prospective cohort study. J. Nutr. 2021;151:2731–2740. doi: 10.1093/jn/nxab158. [DOI] [PubMed] [Google Scholar]
- 24.Thompson A.S., Tresserra-Rimbau A., Karavasiloglou N., Jennings A., Cantwell M., Hill C., Perez-Cornago A., Bondonno N.P., Murphy N., Rohrmann S., et al. Association of healthful plant-based diet adherence with risk of mortality and major chronic diseases among adults in the UK. JAMA Netw. Open. 2023;6:e234714. doi: 10.1001/jamanetworkopen.2023.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K., Kim H., Rebholz C., Kim J. Association between different types of plant-based diets and risk of dyslipidemia: A prospective cohort study. Nutrients. 2021;13:220. doi: 10.3390/nu13010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luque V., Closa-Monasterolo R., Grote V., Ambrosini G.L., Zaragoza-Jordana M., Ferré N., Theurich M., Koletzko B., Verduci E., Gruszfeld D., et al. Dietary patterns acquired in early life are associated with cardiometabolic markers at school age. Clin. Nutr. 2021;40:4606–4614. doi: 10.1016/j.clnu.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Mikkilä V., Räsänen L., Raitakari O.T., Pietinen P., Viikari J. Consistent dietary patterns identified from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr. 2005;93:923–931. doi: 10.1079/BJN20051418. [DOI] [PubMed] [Google Scholar]
- 28.Wallace A., Kirkpatrick S.I., Darlington G., Haines J. Accuracy of parental reporting of preschoolers' dietary intake using an online self-administered 24-h recall. Nutrients. 2018;10:987. doi: 10.3390/nu10080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health Canada Nutrition Labelling: Table of Reference Amounts for Food. 2016. [(accessed on 14 June 2023)]. Available online: https://www.canada.ca/content/dam/hc-sc/healthy-canadians/migration/eating-nutrition/label-etiquetage/regulatory-guidance-directives-reglementaires/reference-amounts-food-quantites-reference-aliments/alt/reference-amounts-food-quantites-reference-aliments-eng.pdf.
- 30.Roberts M., Tolar-Peterson T., Reynolds A., Wall C., Reeder N., Rico Mendez G. The effects of nutritional interventions on the cognitive development of preschool-age children: A systematic review. Nutrients. 2022;14:532. doi: 10.3390/nu14030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G., Su M., Chu X., Wei Y., Chen S., Zhou Y., Liu Z., Zhang Z. Plant-based diets and body composition in Chinese omnivorous children aged 6–9 years old: A cross-sectional study. Front Nutr. 2022;9:918944. doi: 10.3389/fnut.2022.918944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asoudeh F., Mousavi S.M., Keshteli A.H., Hasani-Ranjbar S., Larijani B., Esmaillzadeh A., Adibi P. The association of plant-based dietary pattern with general and abdominal obesity: A large cross-sectional study. J. Diabetes Met. Disord. 2023;22:469–477. doi: 10.1007/s40200-022-01166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamani B., Daneshzad E., Siassi F., Guilani B., Bellissimo N., Azadbakht L. Association of plant-based dietary patterns with psychological profile and obesity in Iranian women. Clin Nutr. 2020;39:1799–1808. doi: 10.1016/j.clnu.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Shirzadi Z., Daneshzad E., Dorosty A., Surkan P.J., Azadbakht L. Associations of plant-based dietary patterns with cardiovascular risk factors in women. J. Cardiovasc. Thorac. Res. 2022;14:1–10. doi: 10.34172/jcvtr.2022.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolori P., Setaysh L., Rasaei N., Jarrahi F., Yekaninejad M.S., Mirzaei K. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women—A cross-sectional study. Diabetes Metab. Syndr. 2019;13:2795–2802. doi: 10.1016/j.dsx.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y., Tan J., Tan Z., Shang L. Validity and reliability of semiquantitative food frequency questionnaires for assessing nutrient intake among preschool children in northwest China. J. Healthc. Eng. 2022;2022:1677252. doi: 10.1155/2022/1677252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox M.K., Condon E., Briefel R.R., Reidy K.C., Deming D.M. Food consumption patterns of young preschoolers: Are they starting off on the right path? J. Am. Diet. Assoc. 2010;110:S52–S59. doi: 10.1016/j.jada.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Dror D.K., Allen L.H. Dairy product intake in children and adolescents in developed countries: Trends, nutritional contribution, and a review of association with health outcomes. Nutr. Rev. 2014;72:68–81. doi: 10.1111/nure.12078. [DOI] [PubMed] [Google Scholar]
- 39.Liu R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013;4:384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papanikolaou Y., Slavin J.L., Clemens R., Brenna J.T., Hayes D., Gaesser G.A., Fulgoni V.L. III. Do refined grains have a place in a healthy dietary pattern: Perspectives from an expert panel consensus meeting. Curr. Dev. Nutr. 2020;4:nzaa125. doi: 10.1093/cdn/nzaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulton S.L., McKinley M.C., Young I.S., Cardwell C.R., Woodside J.V. The effect of increasing fruit and vegetable consumption on overall diet: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2016;56:802–816. doi: 10.1080/10408398.2012.727917. [DOI] [PubMed] [Google Scholar]
- 42.Mead L.C., Hill A.M., Carter S., Coates A.M. The effect of nut consumption on diet quality, cardiometabolic and gastrointestinal health in children: A systematic review of randomized controlled trials. Int. J. Environ. Res. Public Health. 2021;18:454. doi: 10.3390/ijerph18020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Interested researchers can contact GFHS investigators to explore data availability in alignment with the University of Guelph Research Ethics Board.