Abstract
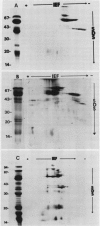
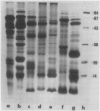
The globulins from wheat caryopses were found to consist primarily of protein sedimenting at approximately 3S and 7S. These proteins displayed a molecular weight distribution similar to that of the purified vicilin-like fractions from oat and pea, with variations occurring in the isoelectric points and relative quantities of their major subunits. concanavalin A Sepharose chromatography suggested that the major polypeptides of the wheat (3S + 7S) fraction are glycosylated. Western blot analysis using antioat (3S + 7S) globulin immunoglobulin G revealed the vicilins from pea and the globulin fractions of oat, wheat, barley, rye, corn, and rice to contain immunologically homologous polypeptides. Major groups of polypeptides were shared by all the cereals and pea, including subunits of approximately 75, 50, 40 kilodaltons and 20 to 25 kilodaltons. These results indicate that legume-like 3S and 7S globulins have been conserved and are being expressed in cereals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeli K., Altosaar I. Characterization of oat vicilin-like polypeptides. Plant Physiol. 1984 May;75(1):225–227. doi: 10.1104/pp.75.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Separation and characterization of oat globulin polypeptides. Arch Biochem Biophys. 1982 Nov;219(1):71–79. doi: 10.1016/0003-9861(82)90135-7. [DOI] [PubMed] [Google Scholar]
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse J. A., Croy R. R., Morton H., Tyler M., Boulter D. Characterisation and subunit structures of the vicilin storage proteins of pea (Pisum sativum L.). Eur J Biochem. 1981 Sep 1;118(3):627–633. doi: 10.1111/j.1432-1033.1981.tb05565.x. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of soybean seeds: I. Isolation and characterization of the major components. Plant Physiol. 1974 May;53(5):742–746. doi: 10.1104/pp.53.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert L. S., Nozzolillo C., Altosaar I. Homology between legumin-like polypeptides from cereals and pea. Biochem J. 1985 Mar 15;226(3):847–852. doi: 10.1042/bj2260847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh V. H., Okubo K., Shibasaki K. Isolation and Characterization of the Multiple 7S Globulins of Soybean Proteins. Plant Physiol. 1975 Jul;56(1):19–22. doi: 10.1104/pp.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburg G., Larkins B. A. Oat seed globulin: subunit characterization and demonstration of its synthesis as a precursor. Plant Physiol. 1983 May;72(1):161–165. doi: 10.1104/pp.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]