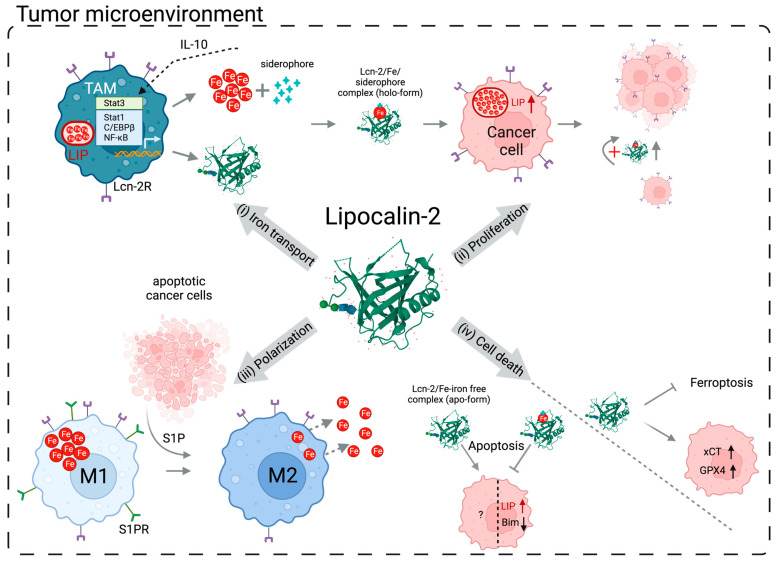
Figure 1.
Overview of Lcn-2’s mode of action and effect on (i) iron transport, (ii) cancer cell proliferation, (iii) macrophage polarization, and (iv) cell death in the TME. (i) Lcn-2 expression induced either via the IL-10/STAT3 axis or via different pro-inflammatory cytokines, and iron export, are upregulated by M2-like TAMs, thereby decreasing the LIP. (ii) Siderophore-bound iron is transported and internalized by cancer cells via Lcn-2 (i.e., holo-Lcn-2) and Lcn-2R (24p3R), respectively. Increased LIP inside cancer cells, among other factors, enables cells to proliferate, whilst holo-Lcn-2 continuously provides iron. (iii) M1- and M2-like macrophages, both present in the TME, throughout the course of tumorigenesis are under the influence of different factors, such as S1P, released by apoptotic cancer cells. Upon the binding of S1P to S1PR, macrophages will acquire an iron-releasing anti-inflammatory phenotype. (iv) Finally, Lcn-2 affects apoptosis and ferroptosis by obtaining different formats, whereby the internalization of apo- versus holo-Lcn-2 regulates cell iron availability. Abbreviations: Lcn-2—Lipocalin-2, TME—tumor microenvironment, IL-10—interleukin 10, STAT3—signal transducer and activator of transcription 3, TAM—tumor-associated macrophage, LIP—labile iron pool, Lcn-2R (24p3R)—Lipocalin-2 receptor, holo-Lcn-2—Lcn-2 bound to iron–siderophore complex, S1P—sphingosine-1-phosphate, S1PR—sphingosine-1-phosphate receptor, and apo-Lcn-2—Lcn-2 not bound to iron–siderophore complex. Image created using BioRender.com.